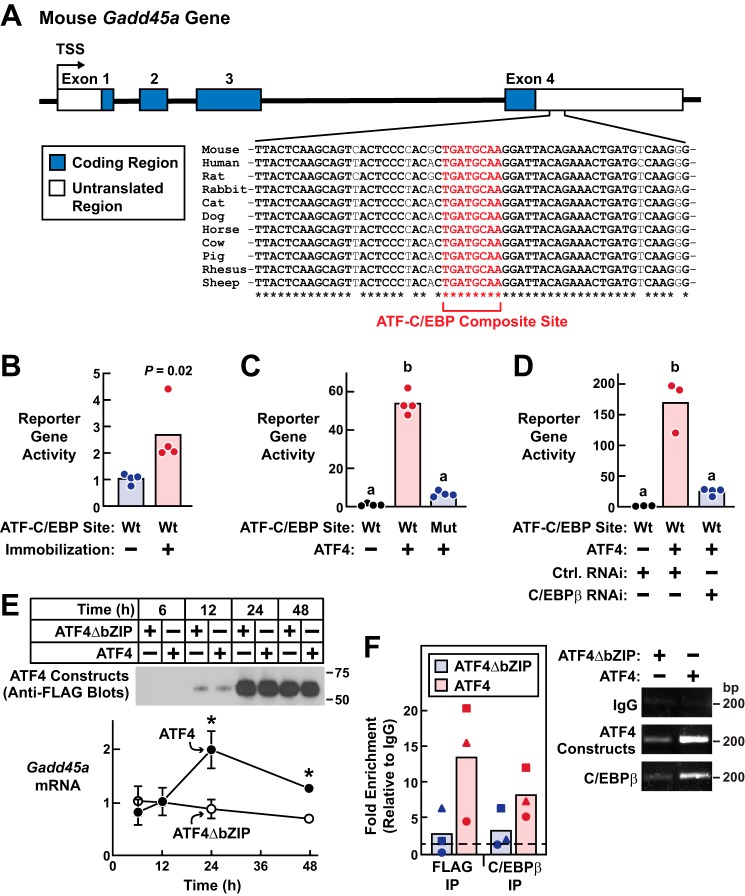
Figure 7.
ATF4–C/EBPβ binds and activates an evolutionarily-conserved regulatory element in the Gadd45a gene. A, schematic illustration of the mouse Gadd45a gene and the conserved ATF–C/EBP composite site in the 3′-untranslated region (UTR) of exon 4. To generate the “WT” reporter plasmid described in B–D, we placed this ATF–C/EBP composite site plus some flanking sequence from the 3′-UTR (+1733 to +1933 bp relative to the Gadd45a transcription start site) upstream of the transcription start site and luciferase-coding region in the pGL3-Basic reporter plasmid. To generate the “mutant” reporter plasmid described in C, we changed the ATF–C/EBP composite site in the WT reporter from TGATGCAA to CAGAATGG. B, bilateral mouse TA muscles were transfected with 5 μg of WT reporter plasmid plus 0.3 μg of Renilla plasmid. Three days after transfection, one hind limb in each mouse was immobilized. Six days post-transfection, bilateral TAs were harvested for analysis of reporter gene activity. In each muscle, the level of reporter gene activity was normalized to the average level in the transfected mobile TAs. Each circle represents one transfected muscle, and bars denote the means. p value was determined with a paired t test. C, mouse TA muscles were transfected with 5 μg of WT reporter plasmid + 5 μg of empty plasmid (pcDNA3) + 0.3 μg of Renilla plasmid (group 1); 5 μg of WT reporter plasmid + 5 μg of ATF4–FLAG plasmid + 0.3 μg of Renilla plasmid (group 2); or 5 μg of mutant reporter plasmid + 5 μg of ATF4–FLAG plasmid + 0.3 μg of Renilla plasmid (group 3), as indicated. TA muscles were harvested 7 days post-transfection for analysis of reporter gene activity, which was then normalized to the average level in group 1. Each circle represents one transfected muscle, and bars denote the means. Different letters denote statistically differences (p ≤ 0.05) by one-way ANOVA with Dunnett's post test. D, mouse TA muscles were transfected with 5 μg of WT reporter plasmid + 5 μg of pcDNA3 + 10 μg of control RNAi plasmid + 0.3 μg of Renilla plasmid (group 1); 5 μg of WT reporter plasmid + 5 μg of ATF4–FLAG plasmid + 10 μg of control RNAi plasmid + 0.3 μg of Renilla plasmid (group 2); or 5 μg of WT reporter plasmid + 5 μg of ATF4–FLAG plasmid + 10 μg of C/EBPβ RNAi plasmid + 0.3 μg of Renilla plasmid (group 3). TA muscles were harvested 7 days post-transfection for analysis of reporter gene activity, which was then normalized to the average level in group 1. Each circle represents one transfected muscle, and bars denote the means. Different letters denote statistically differences (p ≤ 0.05) by one-way ANOVA with Dunnett's post test. E, fully differentiated C2C12 myotubes were treated with recombinant adenoviruses expressing either ATF4–FLAG or a full-length transcriptionally inactive ATF4–FLAG construct (ATF4ΔbZIP) and then harvested at the indicated times post-adenovirus treatment for immunoblot analysis using HRP-conjugated mouse monoclonal anti-FLAG IgG (top) and qPCR analysis of Gadd45a mRNA (bottom). In the qPCR analysis, data points are means ± S.D. from three replicates/condition. Some error bars are too small to see. *, p ≤ 0.05 relative to ATF4ΔbZIP. F, fully-differentiated C2C12 myotubes were treated as in E and harvested 24 h after adenovirus treatment. ChIP was then performed using either control anti-IgG, anti-FLAG, or anti-C/EBPβ, followed by qPCR analysis of the same exon 4 Gadd45a sequence used in the WT reporter plasmid described in A–D, left, results from three independent experiments. Each circle, triangle, or square represents data from one independent experiment; data from the same experiment were assigned the same symbols. Relative to IgG, fold enrichment was significantly increased in the ATF4 but not ATF4ΔbZIP samples (p < 0.05 by one-way ANOVA with Dunnett's post test). Right, representative agarose gel images.