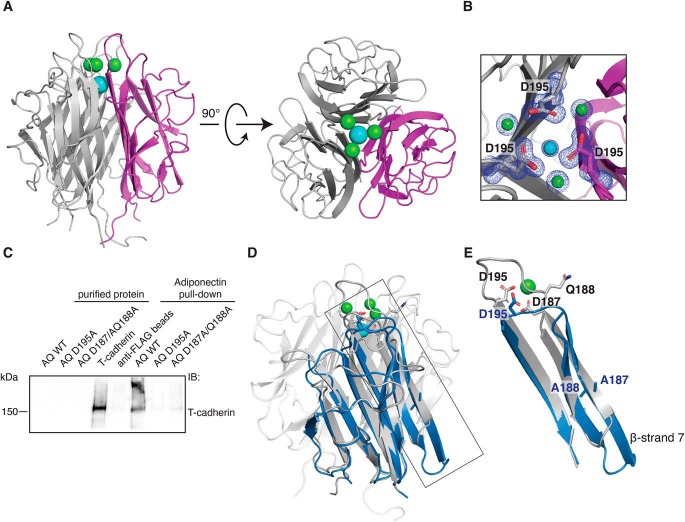
Figure 2.
Organization of the metal ion binding site is necessary for T-cadherin binding. A, crystal structure of the WT adiponectin globular domain. The protein self-assembles as a trimer; one monomer is colored magenta. Three calcium ions are depicted in green, and a single sodium ion is shown in cyan. B, close-up view of the metal ion coordination site. The 2Fo-Fc map is shown in blue and is contoured at 1 σ. C, FLAG-adiponectin pulldown assay showing that T-cadherin cannot be pulled down efficiently by mutant forms of adiponectin (abbreviated AQ for AdipoQ) in which the metal ion binding site is disrupted. D, superimposition of adiponectin D187A/Q188A (blue) on the WT structure (gray). The side chains of the mutated amino acids Asp-187 and Gln-188 are shown in both structures, together with Asp-195. E, close-up of the region affected by mutations. The superimposition shows a major change in the conformation of β-strand 7 and in loop 7.