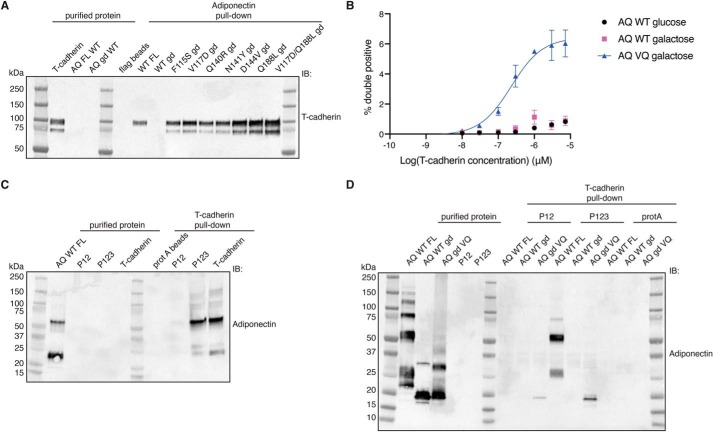
Figure 6.
Mutations in the globular domain confer binding to T-cadherin in vitro. A, FLAG pulldown assay. Purified FLAG-adiponectin proteins were immobilized on FLAG resin and then incubated with T-cadherin. Binding of T-cadherin was assessed by Western blotting. IB, immunoblot. B, dose–response binding assay in yeast by flow cytometry. Test proteins (adiponectin globular domain and mutant) were expressed under the control of galactose induction. Data are shown as means ± S.D. from two independent biological replicates, each performed in triplicate. On the y axis, the percentage of cells positive for both the HA epitope (to confirm display) and T-cadherin binding is shown. C, protein A pulldown assay. Purified T-cadherin constructs fused with human IgG1 Fc were immobilized on protein A resin and then incubated with adiponectin. Binding of adiponectin was assessed by Western blotting. D, protein A pulldown assay. Purified T-cadherin constructs fused with Fc were immobilized on protein A resin and then incubated with different adiponectin proteins. Binding of adiponectin was assessed by Western blotting. All pulldown data are representative of experiments performed at least in duplicate.