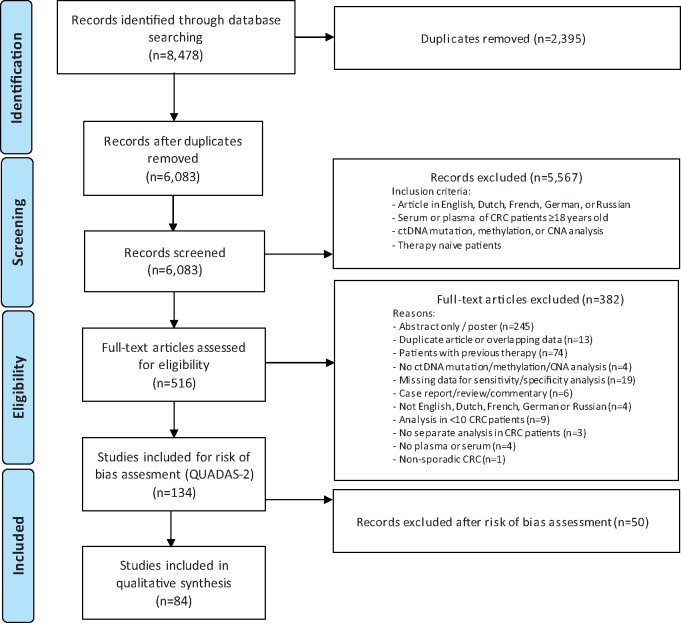
Figure 2.
The Preferred Reporting Items for Systematic Reviews and Meta-analyses flowchart for inclusion of the studies. The risk of bias assessment using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) was incorporated in the flowchart. CNA = copy number alteration; CRC = colorectal cancer; ctDNA = circulating tumor DNA.