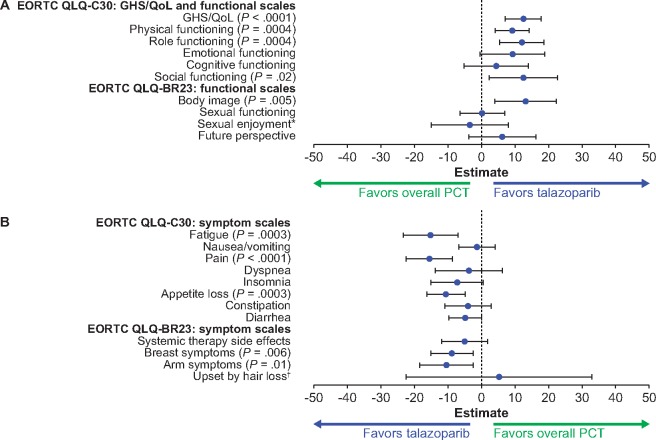
Figure 4.
PROs for the TNBC population. A) EORTC QLQ-C30: global health status or quality of life (GHS/QoL) and functional scales; EORTC QLQ-BR23: functional scales. B) EORTC QLQ-C30: symptom scales; EORTC QLQ-BR23: symptom scales. Forest plot model of estimated difference (talazoparib and overall PCT) in overall change from baseline (repeated measures mixed-effect model) in PRO-evaluable population (P values are shown only if significant between-arm differences, P < .05, were observed). *The sample sizes for the “sexual enjoyment” functional scale were smaller vs other functional scales because patients were asked to respond to this question only if they responded that they were sexually active in a previous question. EORTC = European Organisation for Research and Treatment of Cancer; HR+ = hormone receptor-positive; HER2− = human epidermal growth factor receptor 2-negative; PCT = physician’s choice of chemotherapy treatment; PRO = patient-reported outcomes; QLQ-BR23 = Quality of Life Questionnaire-Breast Cancer Module; QLQ-C30 = Quality of Life Questionnaire-Core 30. †The sample sizes for the “upset by hair loss” symptom scale were smaller vs other symptom scales because patients were asked to respond to this question only if they responded to experiencing hair loss in a previous question. PRO-evaluable population defined as all patients who completed one or more PRO question at baseline and one or more PRO question postbaseline.