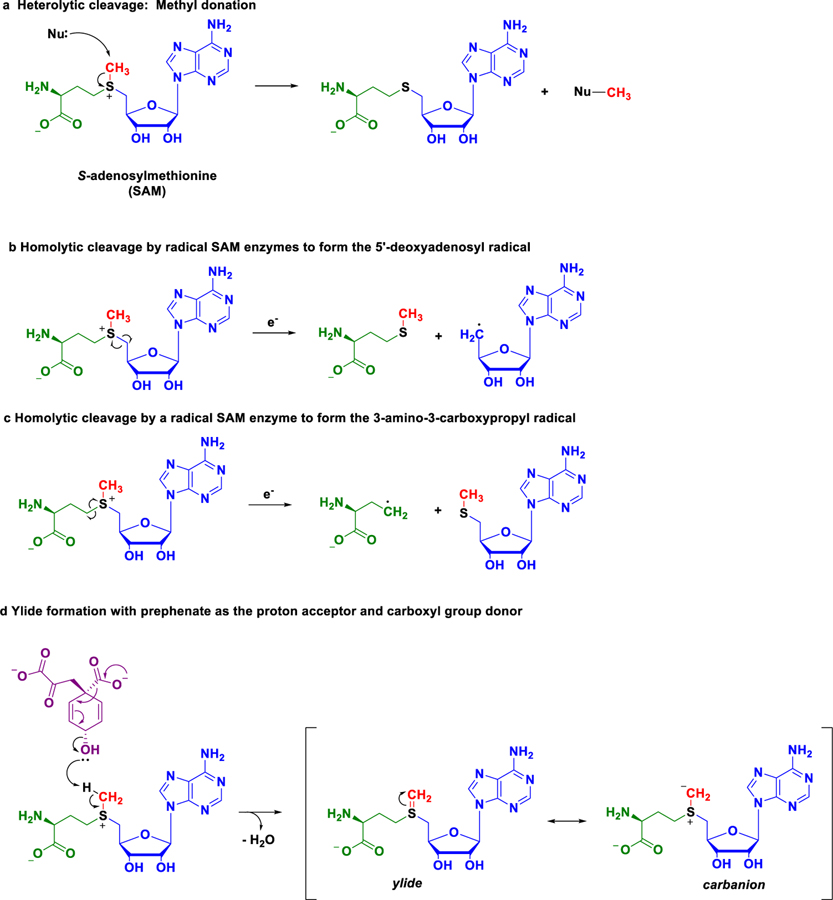
Figure 1. Modes of reactivity for an enzyme co-substrate.
Enzymes commonly use S-adenosylmethionine (SAM) as a co-substrate during catalysis. SAM contains a positively charged sulphur atom bonded to a methyl group (red), a 5’-deoxyadenosyl group (blue), and a 3-amino-3-carboxypropyl group (green). a, The most commonly observed enzyme-catalyzed reaction of SAM in which the sulphur-carbon (S-C) bond to the methyl group is broken. The heterolytic cleavage of the bond results in both of the electrons residing on the sulphur atom. Curled arrows indicate the movement of pairs of electrons, and Nu represents a nucleophile (an electron pair donor). b and c, Radical SAM enzymes use SAM and an electron (e-) to form (b) the 5’-deoxyadenosyl radical or (c) the 3-amino-3-carboxypropyl radical by breaking their respective S-C bonds. The homolytic cleavage of these S-C bonds results in the sulphur and carbon atoms each receiving one electron. Fish hook arrows represent the movement of one electron. d, The findings of Kim et al. show the enzyme CmoA catalyzes a novel reaction mode for SAM with the molecule prephenate (violet). Prephenate acts as a base (proton acceptor) to remove a proton from the methyl group of SAM to form a sulphur-carbon double bond (S=C), resulting in a structure called an ylide (pronounced ill-id). The ylide is represented as two structures, called resonance structures (denoted by brackets), which differ only by the location of electrons on the molecule. The resonance structure of the ylide is a carbanion, whereby the electron pair from the S=C is localized on the carbon atom. The formation of the ylide completely transforms the reactivity of the methyl group carbon atom from electrophilic (electron-pair acceptor) to nucleophilic (electron-pair donor), which the enzyme CmoA facilitates and exploits, in conjunction with CmoB, in the biosynthesis of 5-oxyacetyluridine.