Figure 3 |. Intra- and inter-module interfaces specifying catalytic architecture for ubiquitin priming of substrate by neddylated CRL1β-TRCP with UBE2D.
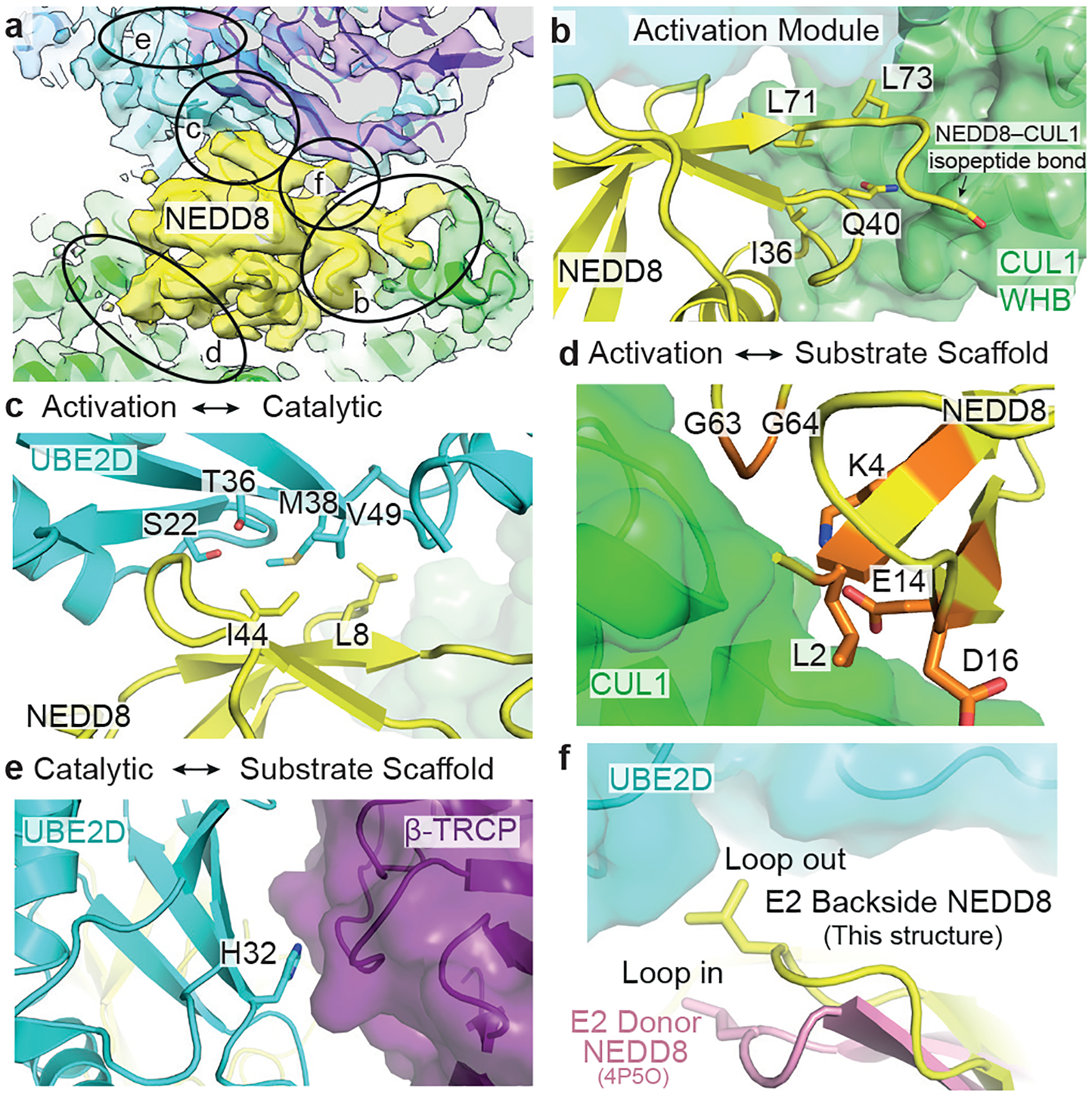
a, Cryo EM density highlighting noncovalent interfaces contributing to the catalytic architecture for neddylated CRL1β-TRCP-mediated UB transfer from UBE2D to a substrate. Circled regions correspond to interfaces within activation module, and between activation and catalytic, activation and substrate scaffolding and catalytic and substrate scaffolding modules shown in panels b-f. b, Close-up of intra-activation module interface, showing NEDD8’s buried polar residue Gln40 and Ile36/Leu71/Leu73 hydrophobic patch making noncovalent interactions with CUL1’s WHB domain adjacent to the isopeptide bond linking NEDD8 and CUL1. c, Close-up of interface between activation and catalytic modules showing key residues at interface between NEDD8 and UBE2D backside. d, Close-up highlighting NEDD8 residues in orange that differ in UB and are at interface with substrate scaffolding module. e, Close-up highlighting UBE2D His32 at interface with substrate scaffolding module. f, Close-up showing role of NEDD8 Loop-out conformation required for binding UBE2D.
