Figure 4 |. Multifarious interactions configuring rapid substrate priming.
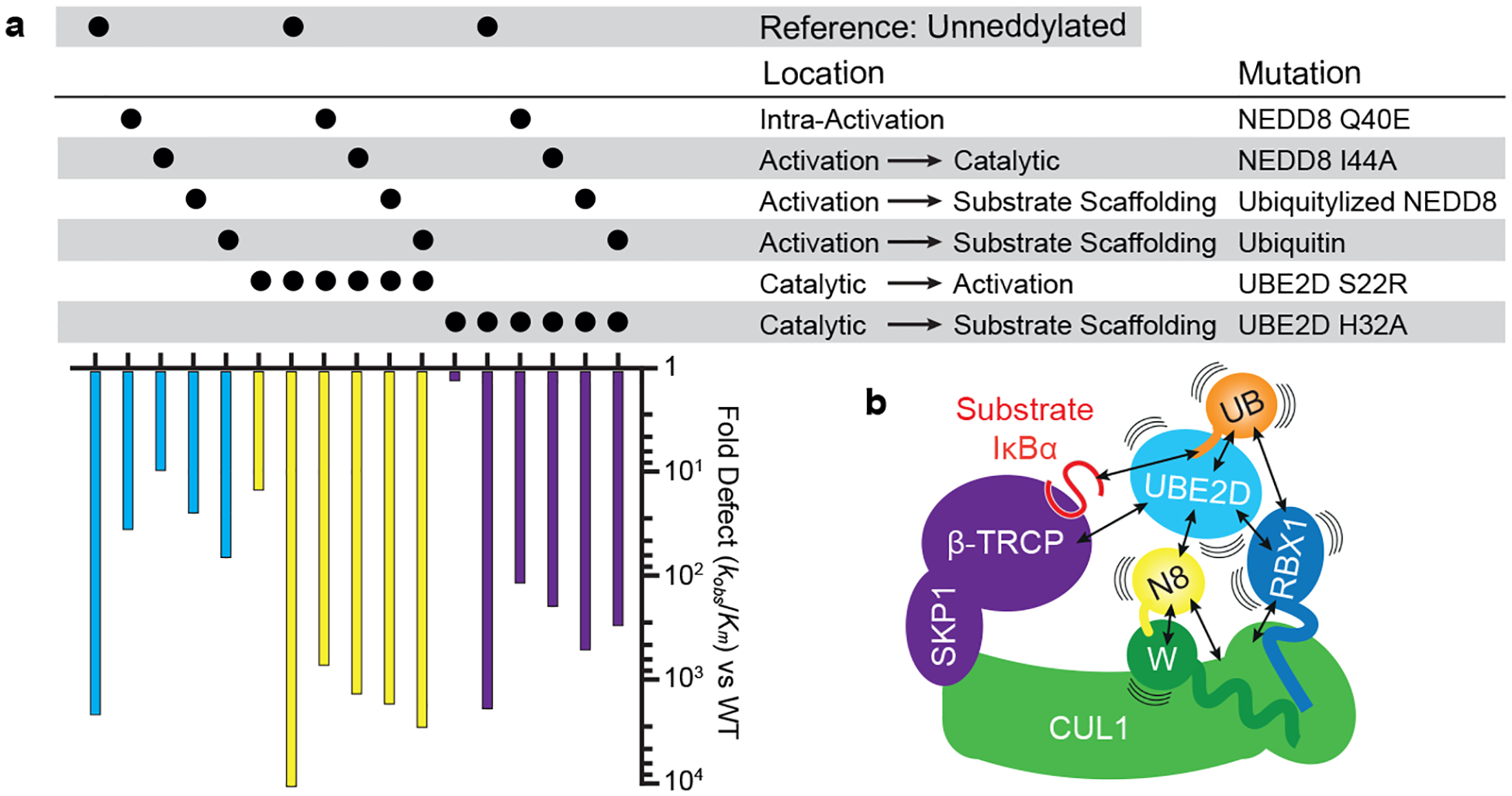
a, Effects of indicated mutants within activation module, between activation and catalytic, activation and substrate scaffolding and substrate scaffolding and catalytic modules, alone or in combination, on catalytic efficiency of substrate priming as quantified by overall fold difference in kobs/Km versus wild-type neddylated CRL1β-TRCP and UBE2D-catalyzed ubiquitylation of a peptide substrate. Reactions with unneddylated CRL1β-TRCP serve as a reference, and used CUL1 K720R to prevent obscuring interpretation of results by artifactual UB transfer to CUL1 and resultant artifactual activation of substrate priming. Graphs show average value from two different experiments (technical replicates), for which curve fits and values are provided in Extended data Figure 1 and Extended Data Table 1. b, On their own, neddylated CRL1β-TRCP and UBE2D~UB are dynamic, and at an extreme their constituent proteins and/or domains may be substantially waving around. Mobile entities are harnessed in the neddylated CRL1β-TRCP-UBE2D~UB-substrate intermediate. There could be multiple routes to the catalytic architecture. It seems equally plausible that the UBE2D~UB intermediate would first encounter RBX1’s RING domain or NEDD8, either of which would raise the effective concentration for the other interaction. Likewise, noncovalent-binding between NEDD8 and its linked WHB domain, or with UBE2D’s backside, would stabilize NEDD8’s Loop-out conformation favoring the other interaction as well. Ultimately, NEDD8, the cullin, and the RBX1-bound UBE2D~UB intermediate make numerous interactions that synergistically establish a distinctive catalytic architecture placing UBE2D adjacent to β-TRCP.
