Extended Data Figure 3 |. Generation of stable proxy for UBE2D~UB-substrate intermediate, and characterization in complexes with neddylated CRL1β-TRCP by cryo EM and biochemistry.
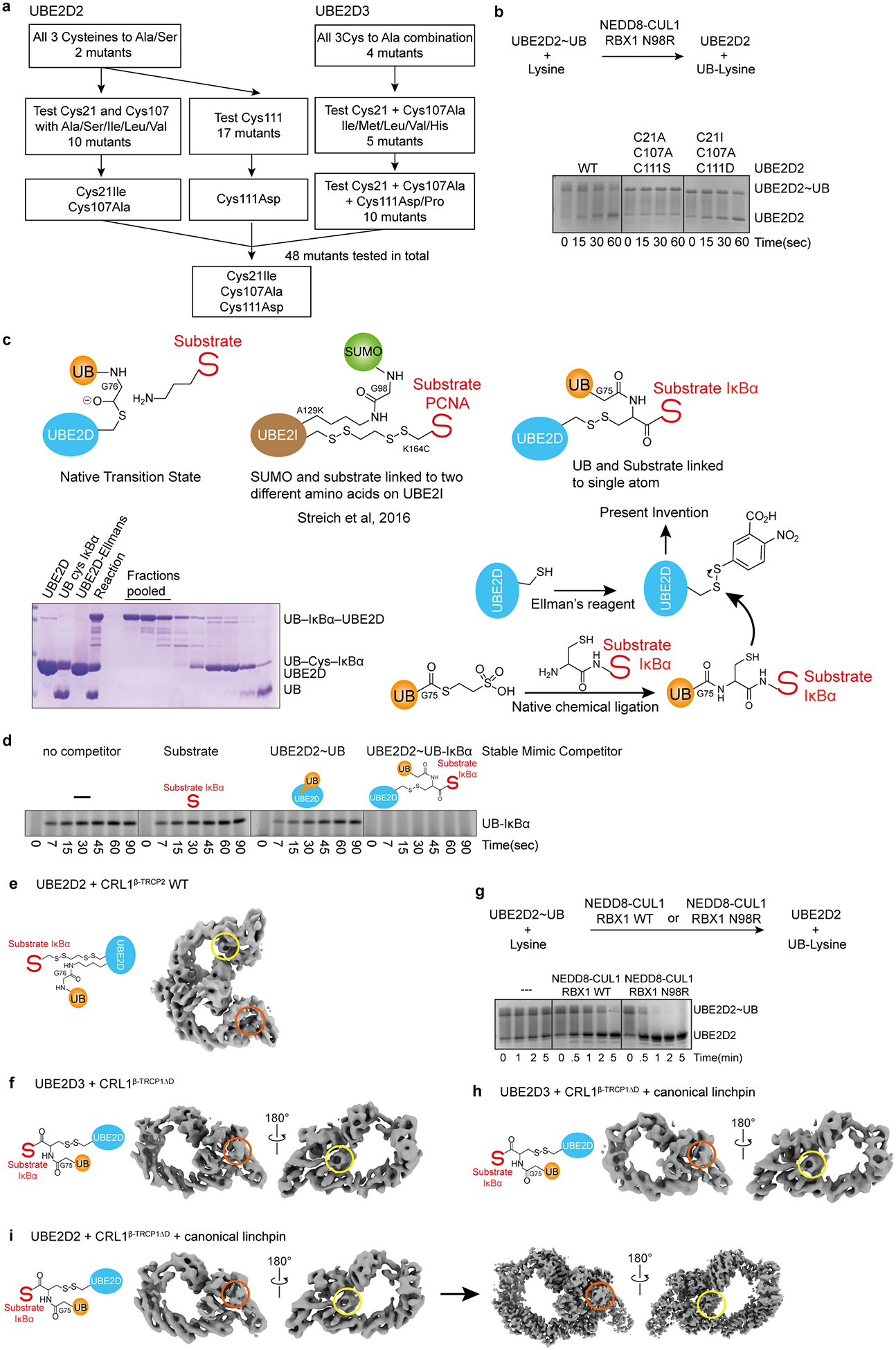
Gel panels in this figure are representative from two independent experiments; n=2.
a, Our strategy for trapping a mimic of the transient neddylated CRL E2~UB-substrate complex requires that the E2 UBE2D contain only a single cysteine at the active site. However, UBE2D contains three additional cysteines (Cys21, Cys107, Cys111). Standard Cys replacements by Ser or Ala severely compromised activity. Based on the structural locations of these cysteines, we presumed that their mutation hindered formation of the RING-activated, closed, active UBE2D~UB conformation28–30. We thus devised a systematic structure- and random-based approach to identify suitable replacements that qualitatively maintain wild-type levels of activity with neddylated CRLs. Structural analysis showed that Cys21 and Cys107 are in close proximity, such that mutation of both residues to Ala may generate a destabilizing cavity at this site. Combining UBE2D2 C107A with Cys21 mutated to Ile, Leu or Val to compensate for the reduced hydrophobic volume led to the identification of C21I C107A as a suitable version for testing of all other possible replacements for Cys111. A similar approach was taken for UBE2D3. A total of 48 different versions of UBE2D were tested to identify the C21I C107A C111D mutant for chemical trapping at the remaining active site Cys. b, Top, schematic of pulse-chase assay testing intrinsic activation of thioester-linked UBE2D~UB intermediates. Although this is often tested by monitoring RING-dependent discharge of UB from UBE2D to free lysine, RBX1 RING-dependent activity is limited in this assay due to sequence constraints imposed by the requirements for binding to partners other than UBE2D39. Nonetheless, substrate-independent activation of UBE2D~UB can be readily visualized using CUL1 complexed with a previously-described hyperactive RBX1 N98R mutant39, and high enzyme and lysine concentrations. UBE2D~UB generated in a pulse reaction was mixed with NEDD8-modified CUL1-RBX1 (shown here with N98R mutant) and free lysine, and UB discharge was monitored over time by Coomassie-stained SDS-PAGE as shown in representative gel on bottom demonstrating that standard Ser/Ala mutations of noncatalytic cysteines compromised activity (shown for C21A C107A C111S), while optimized version (C21I C107A C111D) retains wild-type like activity. c, Overview of the generation of our stable proxy for the phosphorylated IκBα substrate intermediate linked at a single atom, and comparison to the prior method employed to visualize non-canonical Lys sumoylation63. d, Experiment validating our stable proxy for the UBE2D~UB-phosphorylated IκBα substrate intermediate linked at a single atom, based on the hypothesis that its simultaneous occupation of the binding sites for the UBE2D~UB intermediate and substrate should result in more potent inhibition of a neddylated CRL1β-TRCP-dependent substrate priming reaction compared to the individual constituents of the complex. e, Cryo EM reconstruction of neddylated CRL1β-TRCP2 (with full-length, dimeric β-TRCP2) bound to a mimic of the UBE2D2~UB-IκBα generated by adapting the method used previously to visualize non-canonical Lys sumoylation63, where UB is isopeptide-bonded to a UBE2D L119K residue substitution, and a substrate Cys replacement for the acceptor is disulfide-bonded to the UBE2D2 catalytic Cys. This EM map visualizes the catalytic architecture of dimeric CRL1β-TRCP2 wherein the dimerization domain agrees well with the prior crystal structure27, and its linked NEDD8 (encircled in yellow) is bound to the backside of UBE2D, but the donor UB (absent from region circled in orange) was not visible, presumably due to inadequacies of the method used to generate this mimic of the catalytic intermediate, in which the UB and substrate are not both simultaneously linked to the UBE2D catalytic Cys. Variations between the two protomers of the dimer also exacerbated sample heterogeneity. f, Cryo EM reconstruction of neddylated CRL1β-TRCP1ΔD (with monomeric version of β-TRCP1, from residue 175 to the C-terminus20) bound to our newly devised proxy for the UBE2D3~UB-IκBα intermediate. The phoshpo-IκBα peptide-substrate-bound β-TRCP-SKP1-CUL1-RBX1-NEDD8-UBE2D portion of this map superimposes with the map for the dimeric complex shown in e, but here the entire complex is visible, including both the NEDD8 (encircled in yellow) and donor UB (encircled in orange). g, To further increase cryo EM sample homogeneity, we considered that the RBX1 RING sequence represents a compromise to meet requirements for its many different catalytic activities achieved with neddylation E2s, various UB carrying enzymes, and regulators including the inhibitor GLMN72. Therefore, we mutationally introduced a second RBX1 linchpin residue (N98R) previously shown to improve neddylated CRL and UBE2D-dependent substrate priming at the expense of other RBX1-dependent functions (e.g. with UBE2M and UBE2R2)39. Shown is a Coommassie-stained SDS-PAGE gel from assay for intrinsic activity of UBE2D~UB, showing enhanced neddylated CRL-dependent activation of discharge to free lysine with the RBX1 N98R mutation. h,i, Cryo EM reconstructions of neddylated CRL1β-TRCP1ΔD with RBX1 N98R bound to our newly devised proxies for the UBE2D3~UB-IκBα and UBE2D2~UB-IκBα intermediates, the latter of which was pursued for high resolution electron microscopy (final reconstruction refined to 3.7Å resolution shown on right).
