Abstract
Background:
The prevalence of asthma and allergic diseases has increased rapidly in urban China since 2000. There has been limited study of associations between home environmental and lifestyle factors with asthma and symptoms of allergic disease in China.
Methods:
In a cross-sectional analysis of 2,214 children in Beijing, we applied a two-step hybrid Least Absolute Shrinkage and Selection Operator (LASSO) algorithm to identify environmental and lifestyle-related factors associated with asthma, rhinitis and wheeze from a wide range of candidates. We used group LASSO to select variables, using cross-validation as the criterion. Effect estimates were then calculated using adaptive LASSO. Model performance was assessed using Area Under the Curve (AUC) values.
Results:
We found a number of environmental and lifestyle-related factors significantly associated with asthma, rhinitis or wheeze, which changed the probability of asthma, rhinitis or wheeze from −5.76% (95%CI: −7.74%, −3.79%) to 27.4% (95%CI: 16.6%, 38.3%). The three factors associated with the largest change in probability of asthma were short birth length, carpeted floor and paternal allergy; for rhinitis they were maternal smoking during pregnancy, paternal allergy and living close to industrial area; and for wheeze they were carpeted floor, short birth length and maternal allergy. Other home environmental risk factors identified were living close to a highway, industrial area or river, sharing bedroom, cooking with gas, furry pets, cockroaches, incense, printer/photocopier, TV, damp, and window condensation in winter. Lifestyle-related risk factors were child caretakers other than parents, and age<3 for the day-care. Other risk factors included use of antibiotics, and mother’s occupation. Major protective factors for wheeze were living in a rural/suburban region, air conditioner use, and mother’s occupation in healthcare.
Conclusions:
Our findings suggest that changes in lifestyle and indoor environments associated with the urbanization and industrialization of China are associated with asthma, rhinitis, and wheeze in children.
Keywords: Home environment, lifestyle, allergy, asthma, LASSO
1. Introduction
Asthma, the most common chronic disease in children, is among the top 20 chronic conditions in the global ranking of childhood disability-adjusted life years, as well as among the top 10 in the mid-childhood ages of 5-14 years (Asher and Pearce, 2014). Asthma and allergy are rapidly increasing in many Chinese cities while the prevalence of childhood asthma and allergy appear to have plateaued in some western countries (Asher et al., 2006; Bai et al., 2010; Lai et al., 2009; Zhang et al., 2013a). A national survey of 438,000 children up to age 14 years in 43 Chinese cities in 2000 found that the prevalence of physician-diagnosed asthma was 1.97% (Chen, 2003). Although this was relatively low compared to the nearly 8.0% in US reported in 2001, it represented a 64% increase compared to a 1990 survey in China (Chen, 2003; Kemp and Kemp, 2001). In 2010, the prevalence of asthma in children from birth through age 14 in urban areas in China was 3.02%. Although this prevalence was still low compared to that in the US (8.4% in 2010) (Akinbami et al., 2012), the rate of increase was much steeper. Asthma in China increased by 52.8% during 2000-2010, over three times the increase of 15.1% in 2001-2010 in the US (Akinbami et al., 2012; Chen et al., 2016).
Allergic rhinitis is common in children with asthma (de Groot et al., 2012; Price, 2010). Concurrent with the increase of childhood asthma in China, allergic rhinitis, and wheeze, a symptom suggestive of asthma, have also been increasing recently among children (Guo et al., 2019). A survey of ten cities in different geographic regions of China found that the prevalence of rhinitis varied from 24.0% to 50.8%, while the prevalence of wheeze varied from 13.9% to 23.7% (Zhang et al., 2013a).
China has experienced great changes due to rapid modernization and urbanization. China’s urban population in 1990 was about 29% urban, and by the end of 2017, it had increased to about 59% (Sun, 2017). Rapid modernization and urbanization brought dramatic changes to living environments and life styles (Zhang et al., 2013b). Tighter construction techniques leading to reduced ventilation, and extensive use of composite materials have resulted in a degrading of indoor air quality (Zhang et al., 2013b). In 2010, a multiple-centre epidemiological study (China, Children, Homes, Health, abbreviated as CCHH) was launched to explore potential home environmentally associated factors for children’s allergic diseases in China. A cross-sectional questionnaire survey of 48,219 children aged 1–8 years old in 10 Chinese cities was conducted during 2010-2012 (Zhang et al., 2013a). Previous CCHH studies (Bu et al., 2016; Deng et al., 2016a, b; Huang et al., 2015; Lynch et al., 1987; Sun and Sundell, 2011a; Wang et al., 2015; Wang et al. 2016; Wang et al., 2017; Qu et al. 2013) reported that several home environmental factors including living close to a main road or highway, new furniture, dampness, and the presence of cockroaches and mosquito/flies in the home were positively associated with asthma and rhinitis in children. However, these previous CCHH studies are limited since wheeze was not assessed and the analyses focused on only one or several environmental and life-style factors (Qu et al., 2013; Wang et al., 2015; Li et al., 2015). For example, Qu et al. (2013) focused on breastfeeding only and adjusted for age, gender and family history; Wang et al. (2015) considered indoor environmental factors only.
In this analysis, we investigate an expanded suite of factors associated with modernization and urbanization. Our aim is to identify those associated with asthma, rhinitis and wheeze in Chinese children in Beijing.
2. Material and methods
2.1. Study population
This survey was performed in 11 of Beijing’s 16 administrative districts (Dongcheng, Xicheng, Chaoyang, Fengtai, Haidian, Shijingshan, Tongzhou, Changping, Daxing, Mengtougou and Fangshan) as described in Qu et al. (2013). The questionnaires were delivered to randomly selected kindergartens from January to May 2011. The response rate was 64.9%: a total of 9,047 questionnaires were distributed by teachers for children to bring home to parents, and one week later, the children returned 5,876 questionnaires to their teachers. The Medical Research Ethical Committee of the School of Public Health, Fudan University, Shanghai, China granted approval for the study, as part of China, Children, Health and Homes (CCHH) Phase I (International Registered Number: IRB00002408&FWA00002399). Each child’s parents provided informed consent.
2.2. Questionnaire
Our questionnaire was based on the Dampness in Buildings and Health (DBH) questionnaire previously used in Sweden, Bulgaria, Singapore, Taiwan, Denmark, South Korea, and the US (An and Yamamoto, 2016; Bornehag et al., 2005; Harving et al., 1993; Naydenov et al., 2008; Sun and Sundell, 2011b; Tham et al., 2007; Yang et al., 1997). We modified some questions to be appropriate for China’s home environments. The questionnaire includes demographic questions about the child and family as well as questions on the child’s and the family’s health, information on the child’s residence, and information concerning lifestyle habits. The entire questionnaire is available in Zhang et al. (2013a).
2.3. Asthma, rhinitis and wheeze
We obtained the following information from the questionnaire reported by parents regarding their child: (1) asthma diagnosed by a doctor (asthma); (2) hay fever or allergic rhinitis diagnosed by a doctor (rhinitis); (3) parent-observed wheezing or whistling in the chest without respiratory infection in the last 12 months (wheeze). In the questionnaire, we asked about any wheeze in the past 12 months, with additional questions on when the wheeze took place (e.g., when having a cold, during exercise, playing or being outdoors, laughing or crying, in contact with furry animals). We excluded children with wheeze reported only with a cold in efforts to account for wheeze unassociated with a respiratory infection (Castro-Rodríguez et al., 2000).
2.4. Candidate factors
Important associations may not have been identified in previous studies due to focus on a limited set of potential risk factors and manual variable selection. In order to overcome this problem, we considered a more comprehensive range of possible explanatory variables associated with China’s modernization and urbanization. Factors which have been demonstrated or suspected to be associated with allergic diseases or associated symptoms were the candidates in our model. Pregnancy (Erkkola et al., 2009) and family health history factors included paternal and maternal allergy, feeding, nutrition in infancy and early childhood, and medicine use (Qu et al., 2013; Allan and Devereux, 2011). Factors related to family residence were dwelling location (Wieringa et al., 1997; Margolis et al., 2009) and construction characteristics including flooring, wall covering and furniture materials, heating, ventilation, and air conditioning; and dwelling-related behaviors such as window opening (Bornehag et al., 2005). Potential factors in the home environment included environmental tobacco smoke, mold, dampness, furry pets (e.g., cat, dog, rabbits, rats), non-furry pets (e.g., fish, bird), air conditioner, air cleaner, TV and computer (Zhang et al., 2013a). Factors related to the family’s socio-economic status and lifestyle included the mother’s occupation; and which persons were caretakers of the child (Lynch et al., 1987). Details for all home environmental and lifestyle factors (n=64) are summarized in Table S1 in the Supplementary Material.
2.5. Statistical methods
We used a two-step hybrid Least Absolute Shrinkage and Selection Operator LASSO algorithm following the strategy described in Meinsausen (2007) and Meier et al. (2008). Specifically, the first step was group LASSO which identified important variables or variable groups, and screened out unimportant ones. In the second step, we used adaptive LASSO to zoom in on the selected variables and determine the effect estimates.
Variable selection
We adjusted for age and gender a priori, including age as a categorical variable. As there are a large number of potential factors associated with each outcome, we used a group LASSO approach for logistic regression (R package “grpreg”). LASSO’s variable selection and regularization method reduces estimate variances by shrinking regression coefficients towards zero (Tibshirani, 1996). Group LASSO is an extension of LASSO that allows pre-specified groups of covariates to be selected or excluded from a model (Yuan and Lin, 2006). Similar to LASSO, group LASSO applies a λ (i.e., the summation of absolute values of all of a vector's components) penalty to the component regression coefficients, which essentially minimizes the sum of squared errors subject to the sum of the absolute values of the coefficients being less than a given value. We used cross-validation (CV) criteria for variable selection (function “cvfit.glasso” in R package “grpreg”).
We used group LASSO instead of plain LASSO or induced smoothed LASSO (Cillufo et al., 2019) as there are a number of environmental and variables in our data that are reported in categories (Table S1). Using group LASSO ensures that all the dummy variables of each categorical variable of interest are included or excluded in the model.
Model regression
After variable selection, we further applied adaptive LASSO (R packages “glmnet ” and “glmpath”) (Zou, 2006) to determine the regression coefficients from the set of predictors selected in the first step. The final model can be expressed as:
| (1) |
where logit represents the adaptive LASSO for logistic regression; Y is whether the observation is of asthma, rhinitis or wheeze; Y=1 represents “Yes” and Y=0 represents “No”; Xi (i=1, 2, …k) represents LASSO selected variables; βi represents the regression coefficient of Xi; β0 is the intercept of the logistic model.
Variances of the regression coefficients with p-values were estimated by a bootstrap-based procedure (Tibshirani, 1996). A p-value of less than 0.05 was accepted as indicating significance.
Missing data
About half of the returned CCHH questionnaires had missing information for at least one factor. The proportions of missing variables ranged from 0.5% to 16.6% (median=3.0%; IQR=2.3%). The sample of respondents who provided completed questionnaires (2,214) is large enough relative to the number of candidate predictors (65) to have sufficient power to discern differences among covariates using a LASSO approach (Tibshirani, 1996). To determine whether bias would result from not including incomplete questionnaires, we compared the relative frequencies of variables in the 2,214 completed questionnaires with those in the excluded dataset (n=5,876-2,214).
Model evaluation
Once β0 and βi (i=1, 2, …k) have been determined, the probabilities for asthma, rhinitis, or wheeze (π) can be calculated by equation (2):
| (2) |
The accuracy of our model equation (1) was estimated by plotting the area under the curve (AUC) of a receiver operating characteristic (ROC) curve, that is, the plot of true positive rate versus false positive rate for each outcome. The AUC represents the probability that the model correctly ranks the pairs (with and without the disease or symptom) of observations (Bradley, 1997). In addition, we used 10-fold cross validation to avoid an over-fitting bias (Efron and Tibshirani, 1997). The original sample was randomly partitioned into 10 equal sized subsamples. One subsample was retained for model validation, and the other 9 subsamples were used as training data. The cross-validation process was repeated 10 times and the estimate was the average of the 10 results. All analyses were performed with R software (R Development Core Team, Version 3.3.1).
3. Results
3.1. Sample characteristics
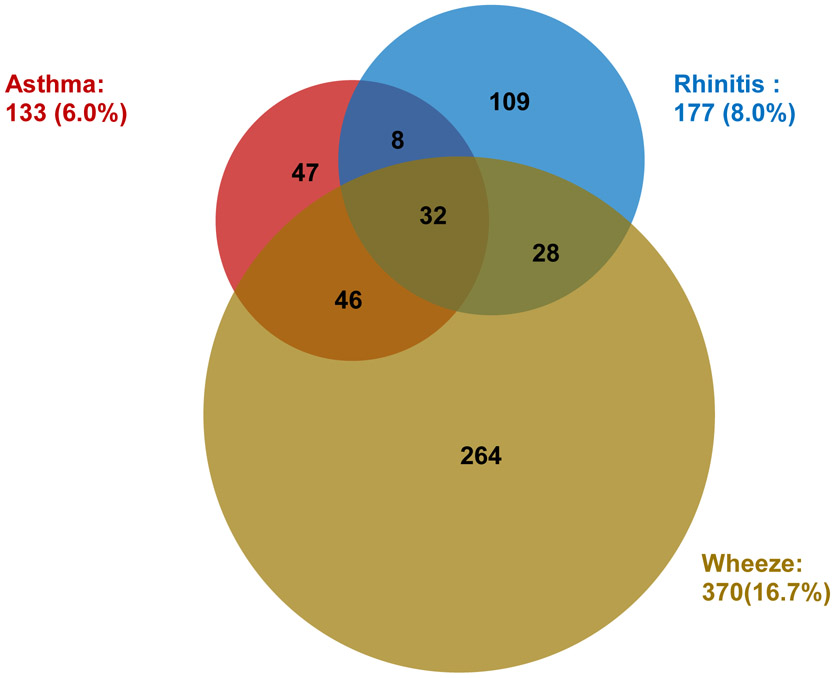
Descriptive information is summarized in Table 1 for 2,214 completed questionnaires. In the analytic sample (n=2,214), the prevalence of asthma, rhinitis and wheeze were 6.0%, 8.0% and 16.7%, and there was overlap among each outcome (Fig 1). Twenty-four percent of the children had at least one of the conditions, and 5.1% had two or three of the conditions.
Table 1.
Demographic and other information for 2214 children
| Variable | n | P (%) | |
|---|---|---|---|
| Asthma | Yes | 133 | 6.0 |
| Rhinitis | Yes | 177 | 8.0 |
| Wheeze | Yes | 370 | 16.7 |
| Gender | Male | 1129 | 51 |
| Female | 1085 | 49 | |
| Age | 3 | 578 | 26.1 |
| 4 | 669 | 30.2 | |
| 5 | 587 | 26.5 | |
| 6 | 379 | 17.1 | |
| Birth weight (kg) | <2 | 20 | 0.9 |
| [2,2.5) | 55 | 2.5 | |
| [2.5,4) | 1895 | 85.6 | |
| [4,5) | 148 | 6.7 | |
| ≥5 | 95 | 4.3 | |
| Birth length (cm) | <40 | 53 | 2.4 |
| [40,46) | 69 | 3.1 | |
| [46,52) | 1665 | 75.2 | |
| [52,58) | 339 | 15.3 | |
| ≥58 | 89 | 4.0 | |
| Family history of allergy | Yes | 6539 | 29.5 |
| Antibiotic | Yes | 1468 | 16.3 |
| House location | urban | 1853 | 83.7 |
| Rural/suburban | 361 | 16.3 |
n is the number; P is the percentage.
Fig. 1.
Prevalence and overlap of asthma, rhinitis and wheeze. The number with each condition and the number that overlap is provided within each circle.
3.2. Associated factors
The regression coefficients and p-values for the LASSO selected variables for asthma, rhinitis and wheeze are summarized in Table S3. We accepted results with p-value<0.05 as significant; and marginally significant when 0.05≤p-value<0.1. Odds ratios of factors significantly associated with at least one of the diseases are presented in Table 2. Significant values are in bold.
Table 2.
Regression results and predicted probability changes for asthma, rhinitis and wheeze based on LASSO selected variables (n = 2214).
| Asthma N=2214 |
Rhinitis N=2214 |
Wheeze N=2214 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | β (95%CI) | p-value | % Difference (95%CI) |
β(95%CI) | p-value | % Difference (95%CI) |
β(95%CI) | p-value | % Difference (95%CI) |
| Gender: male (ref=female) | 0.49 (0.09, 0.89) | 0.016* | 3.07 (2.36, 3.78) | 0.43 (0.10, 0.76) | 0.012* | 3.54 (2.77, 4.32) | 0.27 (0.02, 0.51) | 0.031* | 4.08 (3.02, 5.13) |
| Age 4 (ref=3) | 0.39 (−0.19, 0.97) | 0.183 | 0.00 (−0.01, 0.00) | 0.64 (0.16, 1.12) | 0.009** | 1.28 (0.39, 2.17) | −0.48(−0.79, −0.17) | 0.002** | −1.68 (−2.80, −0.57) |
| Age 5 (ref=3) | 0.41 (−0.17, 1.00) | 0.171 | 0.01 (0.00, 0.01) | 0.57 (0.07, 1.06) | 0.024* | 1.31 (0.34, 2.28) | −0.62(−0.95, −0.29) | <0.001*** | −2.22 (−3.42, −1.02) |
| Age 6 (ref=3) | 0.54 (0.08, 1.00) | 0.022* | 3.52 (2.32, 4.71) | 0.60 (0.07, 1.14) | 0.028* | 1.36 (0.31, 2.41) | −0.66(−1.05, −0.28) | <0.001*** | −4.47 (−5.65, −3.28) |
| Maternal smoking during pregnancy: yes (ref=no) | - | - | - | 2.25 (0.24, 4.25) | 0.029* | 25.4 (2.59, 53.3) | - | - | - |
| Birth season: summer (ref=winter) | - | - | - | −0.49(−0.92, −0.05) | 0.026* | −3.44 (−4.22, −2.66) | - | - | - |
| Birth length (cm): <40 (ref=[46, 52)) | 1.18 (0.22, 2.14) | 0.016* | 18.9 (10.9, 26.9) | 0.79 (−0.04, 1.62) | 0.061 | 18.8 (11.4, 26.2) | 0.64 (−0.05, 1.35) | 0.069 | 15.1 (9.18, 21.0) |
| Birth weight (kg): <2.5 (ref=[2.5, 4)) | 0.64 (−0.26, 1.54) | 0.161 | 8.85 (4.42, 13.3) | - | - | - | 0.78 (0.09, 1.46) | 0.026* | 14.0 (8.17, 19.7) |
| Family history of allergy: yes(ref=no) | 0.96 (0.57, 1.36) | <0.001*** | 7.96 (6.90, 9.03) | 1.20 (0.87, 1.54) | <0.001*** | 11.0 (10.0, 12.1) | 0.52 (0.27, 0.78) | 0.031* | 9.42 (8.09, 10.8) |
| Caretakers other than parents: yes (ref=no) | 0.53 (0.12, 0.94) | 0.011* | 2.90 (2.20, 3.61) | - | - | - | - | - | - |
| Day-care < 3 years old: yes (ref=no) | 0.56 (0.15, 0.96) | 0.007** | 3.57 (2.62, 4.52) | - | - | - | 0.12 (−0.14, 0.40) | 0.356 | 10.0 (9.10, 10.9) |
| Ever antibiotics use: yes (ref= no) | 1.65 (0.98, 2.32) | <0.001 | 6.54 (6.02, 7.07) | 0.71 (0.29, 1.12) | <0.001*** | 5.68 (6.02, 6.34) | 0.84 (0.54, 1.13) | <0.001*** | 10.0 (9.10, 10.9) |
| Rural/suburban (ref=urban) | - | - | - | - | - | - | −0.48(−0.81, −0.14) | 0.005** | −4.47 (−6.10, −2.83) |
| Living close to highway: yes (ref=no) | 0.61 (0.22, 1.01) | 0.002** | 3.58 (2.79, 4.36) | 0.40 (0.07, 0.73) | 0.018* | 3.60 (2.76, 4.43) | 0.23 (−0.02, 0.47) | 0.067 | 3.61 (2.51, 4.71) |
| Living close to industrial area: yes (ref=no) | 0.48 (−0.68, 1.64) | 0.413 | 0.05 (0.00, 0.09) | 1.30 (0.41, 3.19) | 0.001** | 14.7 (8.82, 20.7) | - | - | - |
| Shared bedroom: yes (ref=no) | - | - | - | - | - | - | 0.59 (0.15, 1.04) | 0.009** | 9.15 (8.11, 10.2) |
| Opening window in summer (0=never, 1=sometimes, 2=often) | −0.12 (−0.44, 0.19) | 0.449 | −0.05 (−0.07, −0.02) | −0.32(−0.60, −0.03) | 0.032* | −7.09 (−9.95, −4.23) | −0.20 (−0.41, 0.01) | 0.061 | −4.41 (−7.18, −1.65) |
| Air conditioner: yes (ref=no) | - | - | - | - | - | - | −0.33 (−0.66, 0.00) | 0.047* | −5.44 (−7.32, −3.55) |
| Cooking: gas (ref=electric) | 0.85 (0.04, 1.65) | 0.039* | 3.53 (2.93, 4.14) | - | - | - | 0.23 (−0.15, 0.62) | 0.229 | 1.98 (0.46, 3.49) |
| Floor: carpet (ref=cement) | 1.54 (0.12, 2.96) | 0.033* | 12.1 (3.80, 20.4) | - | - | - | 1.63 (0.51, 2.74) | 0.004** | 24.6 (13.2, 36.1) |
| Pets: yes (ref=no) | 0.48 (0.06, 0.90) | 0.026* | 3.68 (2.55, 4.80) | 0.42 (0.06, 0.77) | 0.023* | 4.65 (3.48, 5.84) | - | - | - |
| Cockroach: yes (ref=no) | 0.20 (−0.08, 0.49) | 0.163 | 0.01 (0.00, 0.16) | - | - | - | 0.21 (0.03, 0.39) | 0.019* | 3.15 (2.04, 4.25) |
| Printer/photocopier in home: yes (ref=no) | - | - | - | 0.50 (0.21, 2.43) | 0.015* | 7.50 (5.67, 9.33) | 0.31 (−0.02, 0.64) | 0.064 | 7.03 (5.09, 8.98) |
|
Air out bedding (0=never, 1=sometimes, 2=often) |
- | - | - | −0.34(−0.03, −0.64) | 0.030* | −2.15 (−0.55, −3.75) | - | - | - |
| Perceived dryness indoor (0=never, 1=sometimes, 2=often) | 0.34 (0.06, 0.62) | 0.019* | 3.05 (2.40, 3.71) | 0.24 (0.01, 0.46) | 0.039* | 3.80 (2.98, 4.62) | - | - | - |
| Perceived bad odor indoor: yes (ref=no) | - | - | - | 0.27 (−0.09, 0.62) | 0.142 | 2.52 (1.72, 3.31) | 0.62 (0.34, 0.90) | <0.001*** | 10.2 (9.26, 11.1) |
| Mother’s occupation: healthcare (ref=housewife) | −0.35 (−0.77, 0.06) | 0.095 | −1.00 (−1.74, −0.26) | −0.74 (−1.69, 0.22) | 0.129 | −2.65 (−3.89, −1.41) | −0.92(−1.64, −0.18) | 0.014* | −7.04 (−8.76, −5.32) |
| TV: yes (ref=no) | - | - | - | - | - | - | 0.30 (0.04, 0.55) | 0.023* | 4.81 (3.66, 5.97) |
Note that eβ is the Odds Ratio. Significant results are in blue bold font; N is the number of observations in the model; “-” represents a variable not selected by LASSO
0.01≤p-value<0.05
0.001≤p-value<0.01
p-value<0.001; % Difference is the difference (%) in predicted probabilities between exposure and reference groups; 95%CI is the 95% confidence interval; ref represents the reference value (no “ref” for ordinal variables).
We found that boys were at higher risk than girls for all three conditions, and significantly so for asthma and rhinitis. The probabilities of asthma and rhinitis increased with age, while the probability of wheeze decreased with age.
Several risk factors were associated with all or with two of the conditions: short birth-length (all), use of antibiotics ever (all), paternal allergy (asthma and rhinitis), maternal allergy (rhinitis and wheeze), living close to highway (asthma and rhinitis), window condensation in winter (rhinitis and wheeze), carpet floor compared to cement floor (asthma and wheeze).
Other factors significantly associated with at least one’ outcome included: day-care <3 years old with asthma (positive and significant) and wheeze (positive and marginally significant); living close to industrial area with rhinitis (positive and significant) and wheeze (positive); gas cooking (compared to electric cooking) with asthma (positive and significant) and wheeze (positive); furry pets with asthma (positive and significant), and rhinitis and wheeze (positive); printer/photocopier in the home with rhinitis (positive and significant) and wheeze (positive).
Other factors associated with at least one outcome included: day-care <3 years old with asthma (positive and significant) and wheeze (positive and marginally significant); living close to industrial area with rhinitis (positive and significant) and wheeze (positive); gas cooking (compared to electric cooking) with asthma (positive and significant) and wheeze (positive); furry pets with asthma (positive and significant), and rhinitis and wheeze (positive); printer/photocopier in the home with rhinitis (positive and significant) and wheeze (positive).
Living in a rural/suburban area (compared to an urban area), shared bedroom, use of air conditioning, presence of cockroach in the home, use of incense, TV in the home, damp for clothing and bedding at home, mother’s occupation in healthcare (compared to housewife) were significantly associated with wheeze, but not with asthma or rhinitis. In contrast, some risk factors for asthma or rhinitis e.g., mother smoking during pregnancy, caretakers other than parents, living close to river, were not associated with wheeze.
3.3. Risk of asthma, rhinitis and wheeze due to associated factors
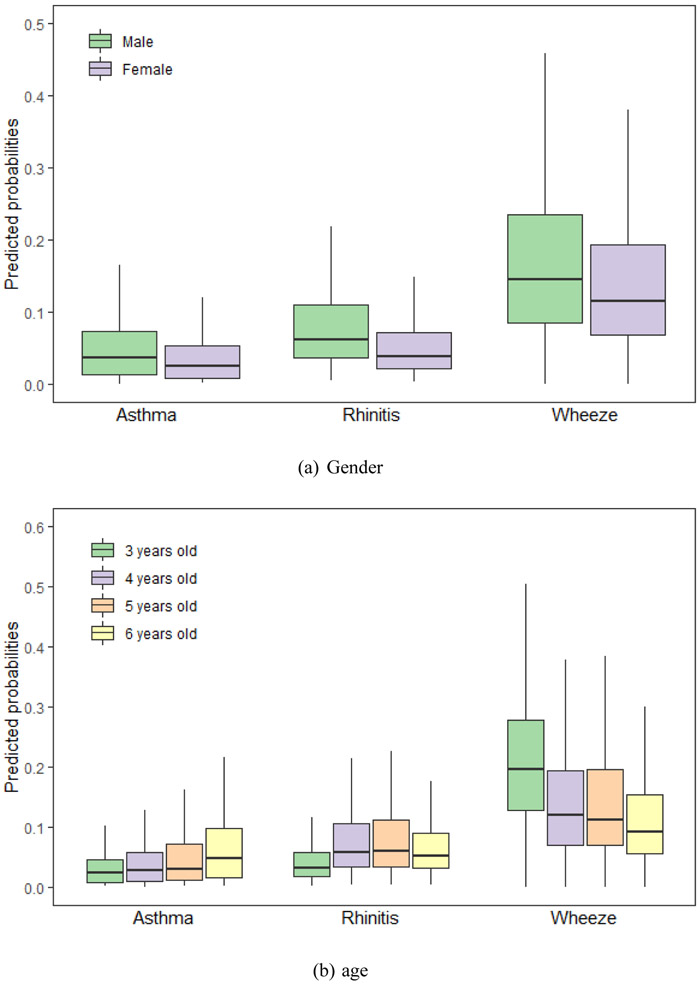
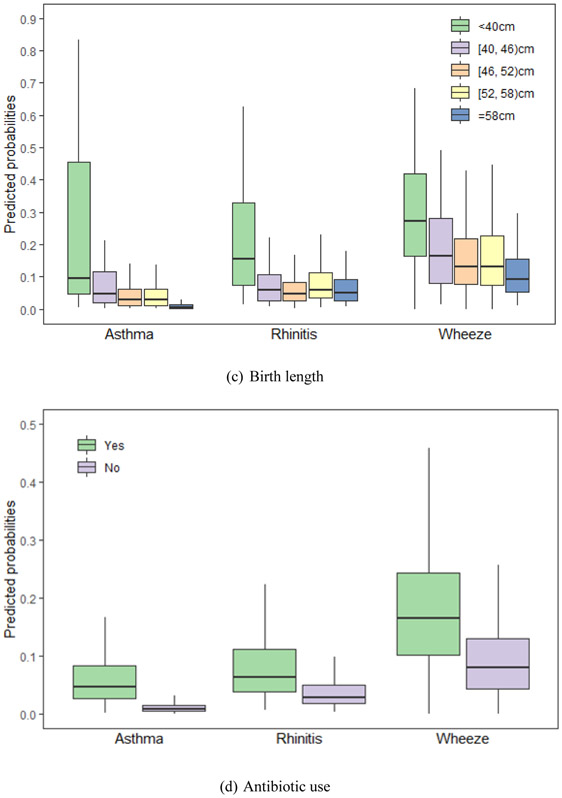
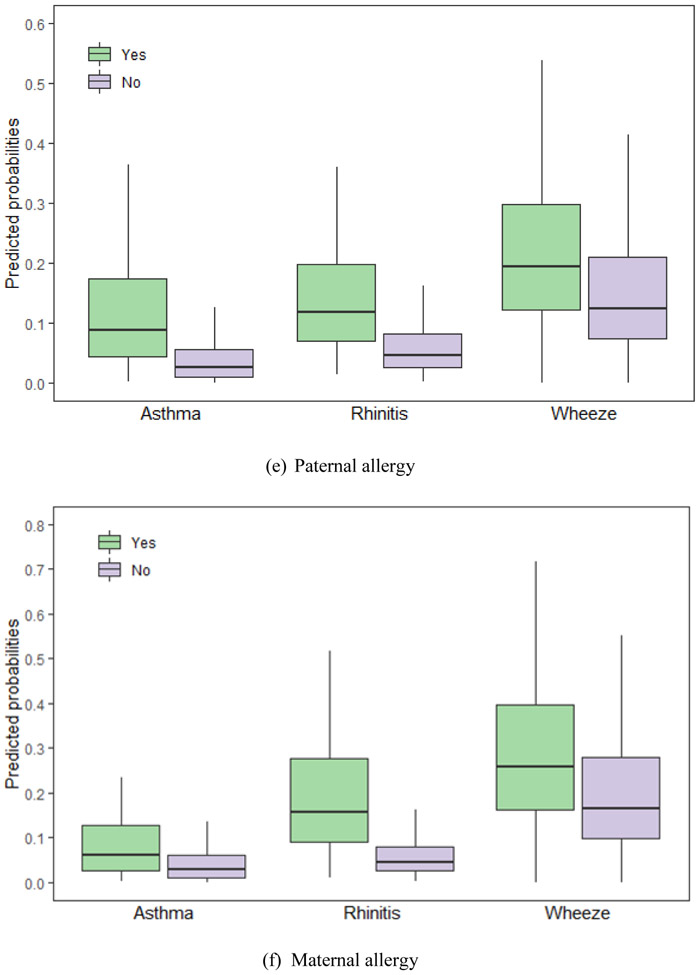
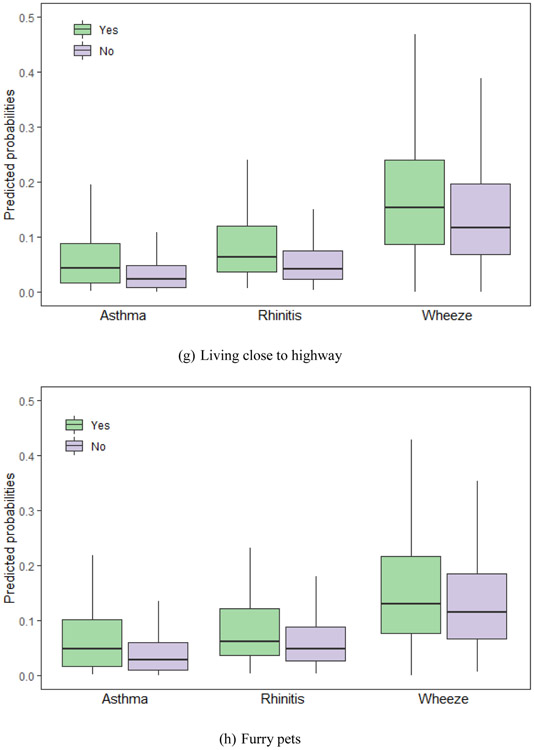
We calculated the probabilities of asthma, rhinitis and wheeze using equation (2) with the LASSO selected factors. Fig. 2 shows predicted probabilities of asthma, and rhinitis and wheeze for common variables selected by LASSO, including gender, age, birth height, antibiotic use, paternal allergy, maternal allergy, living close to highway and furry pets.
Fig. 2.
Predicted probabilities of asthma, rhinitis and wheeze for the Lasso selected risks. The risks are (a) gender, (b) age, (c) birth height, (d) antibiotic use, (e) paternal allergy, (f) maternal allergy, (g) living close to highway and (h) furry pets. (a) Gender. (b) age. (c) Birth length. (d) Antibiotic use. (e) Paternal allergy. (f) Maternal allergy. (g) Living close to highway. (h) Furry pets. Footnote: Only variables selected by group LASSO for each disease – asthma, rhinitis and wheeze – are included. The horizontal lines represent median values and boxes represent 25th-75th percentile range, and the whiskers extend to the 5th and 95th percentiles.
We summarized probability changes due to associated factors in Table 2. Our results show that these home environmental and lifestyle associated factors increased the probability of asthma, rhinitis, or wheeze from −5.8% (95%CI: −7.7%, −3.8%) to 27.4% (95%CI: 16.6%, 38.3%). For example, maternal smoking during pregnancy increased the probability of rhinitis by 25.6% (95CI%: 8.3%, 43.1%); carpeted floor increased the probability of asthma by 13.4% (95CI: 4.3%, 22.4%) compared to cement floor, and 27.4% (95%CI: 16.6%, 38.3%) for wheeze; antibiotics use ever increased the probabilities of asthma, rhinitis and wheeze by 6.3% (95CI%: 5.8, 6.8), 5.4% (95CI%: 4.8%, 6.0%) and 9.5% (95CI%: 8.6%, 10.4%) respectively.
3.4. Bias due to missing
The were no significant difference of relative frequencies of the three outcomes and risk/protective factors between included questionnaires (n=2,214) and the excluded questionnaires (n=5,876-2,214) (most of the p-values of Welch’s two-sample t-test were greater than 0.05) (Table S2 in Supplementary Material), affirming that the missingness was random (Welch, 1947). Therefore, bias was not introduced by using the subset of questionnaires with no missing data.
3.5. Model strength
To show the predictive strength of the factors selected by LASSO, the ROC curves for asthma, rhinitis, and wheeze are provided in the Supplementary Materials Fig. S1. An AUC ≥ 0.70 is generally accepted as indicating a meaningful model (Godil et al., 2013). The AUCs were 0.81 for asthma, 0.77 for rhinitis and 0.75 for wheeze, indicating strong model predictions and associations.
4. Discussion
For 2,214 completed questionnaires, we first used group LASSO to select the most important factors associated with asthma, rhinitis and wheeze for children aged 3-6 in Beijing. We then used adaptive LASSO regression to determine associations between selected factors and these allergies.
We found that the prevalence of asthma in 2010-2011 among children aged 3-6 in Beijing was 6.0%, an increase compared to earlier surveys (Ma et al., 2002; NCGCA, 1993; Zhao et al., 2010). The prevalence of wheeze in the absence of a respiratory infection within the previous 12 months was 16.7%, or slightly higher than the 12.3% reported by Zhao et al. (2010). In contrast, the prevalence (8.0%) of rhinitis in the present study was lower than the 14.9% reported by Zhang et al. (2013c). It is possible that the finding by Zhang et al. (2013c) is attributable to the use of medical record review that included diagnostic tests for the assessment of allergic symptoms, while our result is from self-reported data.
Our results show that asthma, rhinitis and wheeze had several common risk factors (e.g., shorter birth length and antibiotic use). While this finding may be consistent with a common etiology (Bjerg et al., 2015), we note that some factors associated with wheeze were not associated with asthma or rhinitis and that the converse is also true. It is possible that differences may be due to a greater prevalence of parent-reported wheeze than doctor-diagnosed disease, or it is possible that children who have asthma or rhinitis may take medicine that controls associated wheeze. It is also possible that some children may have had asthma or rhinitis, but were not examined by a doctor. In addition, the diagnosis of asthma in younger children is not as reliable as at older ages, as many children with recurrent asthma-like symptoms no longer experience symptoms by age 6 years (van de Kant et al., 2009). All the above possibilities may contribute to uncertainties in the assessment of our outcomes.
To the best of our knowledge, we are the first to describe the positive association between caretakers other than parents with asthma. One past study has suggested a causal relationship between positive parenting attitude and less childhood asthma (Nagano et al., 2010). Modernization in China impacts family structure and relationships (Xu and Xia, 2014). It is also noteworthy that attending a day-care nursery at <3 years old had a significant positive association with asthma, a finding that agrees with Sun and Sundell’s (2011a) study in Northeast Texas.
Early antibiotic use has been reported to be positively associated with allergy (Lapin et al., 2014; Marra et al., 2009; Risnes et al., 2011). China historically has overused antibiotics with physicians commonly prescribing them to as many as half of all outpatients (Hvistendahl, 2012). We note that worsening asthma may be associated with some infections (Custovic et al., 2005); however, it is also possible that doctors prescribe antibiotics in response to non-specific signs of either allergy or infection.
Consistent with many previous studies conducted both inside and outside of China (Beasley, 1998; Pearce et al., 2007; Wang et al., 2015; Qu et al., 2013; Xu et al. 2014), we found that male gender, age, and birth length were significantly associated with asthma, rhinitis and wheeze. We found that paternal allergy was positively and significantly related to asthma and rhinitis, while maternal allergy was positively and significantly related to rhinitis and wheeze, which have been found in previous studies (Litonjua et al., 1997; Paaso et al., 2014). Home environmental factors, including dwelling location and in-dwelling exposure factors and ventilation were also related to asthma, rhinitis or wheeze, similar to previous reports (Li et al., 2013; Margolis et al., 2009; Patel et al., 2011; Vastardi et al., 2012; Lee et al., 2013; Litonjua et al., 2001; Bu et al., 2016; Andersen et al., 1974; Naclerio et al., 1995; Nielsen, 1991; Strom-Tejsen et al., 2008; Sundell and Lindvall, 1993). A recent study in Italy (Cilluffo et al., 2018) found that children living in an urbanized setting had a higher symptom score for symptoms such as red or itching eyes, itching nose, and wheezing compared to children in a less urbanized environment. Between 1978 and 2016, more than 550 million Chinese people have moved to urban from rural/suburban areas because of rapid industrialization (Zhang, 2017). Rapid urbanization has resulted in a 2.6 % decrease of vegetated areas in urban locations per decade, and a 1.5% decrease in suburban areas in China during 1998-2012 (Jin et al., 2018). The changes may have contributed the increase of children’s asthma and allergies in China. A home environmental factor significantly protective against wheeze was air conditioner use, consistent with less exposure to outdoor allergens (Solomon et al., 1980).
In contrast to previous CCHH studies (Bu et al., 2016; Liu et al., 2013; Wang et al., 2015), we found no significant protective association between breastfeeding and no significant risk association between environmental tobacco smoke (ETS) and allergic diseases or symptoms in our study. Associations of both breastfeeding and ETS with allergies are inconsistent in previous studies (Qu et al., 2013; Arif and Racine, 2017; Lebowitz and Quackenboss, 1990; Leung et al., 2016; Lodge et al., 2015; Miyake et al., 2008; Vargas et al., 2007). We found that maternal smoking during pregnancy was significantly associated with rhinitis, consistent with reports from Ferrante et al. (2014) and Kulig et al. (1999). According to Xu et al’s (2017) investigation, the prevalence of maternal smoking in 5 provinces in China is 3.8%. The prevalence of smoking among young women has increased and is predicted to keep increasing due to China’s economic growth and growing independence of women (Snansone et al., 2015).
Our study has a number of strengths. First, we considered a comprehensive suite of candidate predictors compared to previous CCHH studies. We included 64 candidate variables that included demographic information on the child and family, home environmental characteristics, and life habits. Secondly, our two-step LASSO approach had strong model predictive performance as evidenced by all AUCs ≥ 0.75. For comparison, we assessed the strength of the risk predictors for rhinitis detected by Wang et al.’s (2015) Beijing CCHH study based on the same dataset. The AUC for Wang et al.’s model was below 0.60. Larger AUC of our model suggests improved risk identification. In addition, as asthma, wheeze and rhinitis may have similar but not exactly the same etiology, we assessed risk factors in common and risk factors that differed for asthma, rhinitis and wheeze, which have not been evaluated in previous CCHH studies.
A main limitation of the present study is that it is based on a questionnaire survey without physical examinations, allergy screening tests, or environmental measurements such as temperature, humidity, levels of allergens and chemicals in the home. There may be recall errors for both children’s health status and exposure history. However, our analysis did indicate that the missing values were randomly distributed, so that we were able to exclude incomplete questionnaires without introducing error. Secondly, exposures to outdoor pollutants (e.g., NO2) or exposures in school/ daycare environment may be potential influencing factors (Deng et al., 2016b; Salo et al., 2009) were not directly considered in our study. However, we did ask questions that can partly characterize outdoor exposure, such as home location and the traffic time very day. Thirdly, we were not able to assess some potential risk factors, such as the family income, which was found to a risk factor for allergies in a previous study (Chen et al., 2018). Finally, we used “in-sample” validation to evaluate the predictive ability of the observed associations; it would be valuable to investigate whether this model can retain high accuracy when it is applied to other data sets or assessed prospectively.
5. Conclusions
Starting with a relatively large comprehensive suite of home environmental and lifestyle factors, we used LASSO to select key factors associated with asthma, rhinitis and wheeze in children based on 2,214 completed questionnaires in Beijing. Indoor environmental factors associated with increased risk of asthma, rhinitis, and wheeze in children include residence in an urban area, near a highway or an industrial area, carpeted floor, gas cooking, perceived indoor odor, perceived dryness, furry pets, cockroaches. Lifestyle-related factors associated with increased risk of asthma, rhinitis, and wheeze in children include antibiotic use, caretakers other than parents, starting day-care before 3 years old, shared bedroom, maternal smoking, and a printer/photocopier and TV in the home. We note that many of the home environmental and lifestyle factors identified have accompanied rapid modernization and urbanization in China.
Supplementary Material
Acknowledgements
This study was made possible by the findings from Natural Science Foundation of China (51420105010 and 51521005), the NIH (R01 ES019853) and USEPA (RD-83479801 and RD-83587201). We thank the kindergarten teachers, children and their parents for participating in the survey.
Funding
Natural Science Foundation of China (51420105010 and 51521005), the NIH (R01 ES019853) and USEPA (RD-83479801 and RD-83587201).
Footnotes
Competing financial interests: The authors do not have any competing financial interests.
Appendix A. Supporting information
Supplementary Material for this article can be found in the online version.
References
- Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X, 2012. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 94, 1–8. [PubMed] [Google Scholar]
- An C, Yamamoto N, 2016. Fungal compositions and diversities on indoor surfaces with visible mold growths in residential buildings in the Seoul Capital Area of South Korea. Indoor Air 26, 714–723. [DOI] [PubMed] [Google Scholar]
- Andersen I, Lundqvis GR, Jensen PL, Proctor DF, 1974. Human response to 78-hour exposure to dry air. Arch. Environ. Health 29, 319–324. [DOI] [PubMed] [Google Scholar]
- Arif AA, Racine EF, 2017. Does longer duration of breastfeeding prevent childhood asthma in low-income families? J. Asthma 54, 600–605. [DOI] [PubMed] [Google Scholar]
- Asher I, Pearce N, 2014. Global burden of asthma among children. Int J Tuberc Lung Dis. 18, 1269–78. [DOI] [PubMed] [Google Scholar]
- Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H, ISAAC Phase Three Study Group, 2006. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 368, 733–743. [DOI] [PubMed] [Google Scholar]
- Bai J, Zhao J, Shen KL, Xiang L, Chen AH, Huang S, Huang Y, Wang JS, Ye RW, 2010. Current trends of the prevalence of childhood asthma in three Chinese cities: A multicenter epidemiological Survey. Biomed. Environ. Sci 23, 453–457. [DOI] [PubMed] [Google Scholar]
- Beasley R, 1998. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet 351, 1225–1232. [PubMed] [Google Scholar]
- Bjerg A, Eriksson J, Ólafsdóttir IS3, Middelveld R, Franklin K, Forsberg B, Larsson K, Torén K, Dahlén SE, Janson C, 2015. The association between asthma and rhinitis is stable over time despite diverging trends in prevalence. Resp. Med 109, 312–319. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, Hagerhed-Engman L, Sigsggard T, Janson S, Aberg N, DBH Study Group, 2005. ‘Dampness’ at home and its association with airway, nose, and skin symptoms among 10,851 preschool children in Sweden: a cross-sectional study. Indoor Air 15, 48–55. [DOI] [PubMed] [Google Scholar]
- Bradley AP, 1997. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern Recogn. 30, 1145–1159. [Google Scholar]
- Bu ZM, Wang LF, Weschler LB, Li BZ, Sundell J, Zhang YP, 2016. Associations between perceptions of odors and dryness and children’s asthma and allergies: A cross-sectional study of home environment in Baotou. Build. Environ 106, 167–174. [Google Scholar]
- Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD, 2000. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 162, 1403–1406. [DOI] [PubMed] [Google Scholar]
- Chen F, Lin Z, Chen R, Norback D, Liu C, Kan H, 2018. The effects of PM2.5 on asthmatic and allergic diseases or symptoms in preschool children of six Chinese cities, based on China, Children, Homes and Health (CCHH) project. Environ Pollut. 232:329–337. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang JW, Li J, 2016. Environmental exposure and genetic predisposition as risk factors for asthma in China. Allergy Asthma Immunol. Res 8, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YZ, 2003. National Cooperation Group on Childhood Asthma. A nationwide survey in China on prevalence of asthma in urban children (in Chinese). Chin. J. Pediatr 41, 123–127. [PubMed] [Google Scholar]
- Cilluffo G, Ferrante G, Fasola S, Montalbano L, Malizia V, Piscini A, Romaniello V, Silvestri M, Stramondo S, Stafoggia M, Ranzi A, Viegi1 G, Grutta SL, 2018. Associations of greenness, greyness and air pollution exposure with children's health: a cross-sectional study in Southern Italy. Environ. Health 17: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilluffo G, Sottile GS, La Grutta S, Muggeo VMR, 2019. The Induced Smoothed lasso: A practical framework for hypothesis testing in high dimensional regression. Statist. Methods Med. Res DOI: 10.1177/0962280219842890. [DOI] [PubMed] [Google Scholar]
- Custovic A, Murray C, Simpson A, 2005. Allergy and infection: understanding their relationship. Allergy 60, 10–13. [DOI] [PubMed] [Google Scholar]
- de Groot EP, Nijkamp A, Duiverman EJ, Brand PLP, 2012. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 67(7):582–7. [DOI] [PubMed] [Google Scholar]
- Deng Q, Lu C, Li Y, Sundell J, Norbäck D, 2016a. Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ Res. 150:119–127. [DOI] [PubMed] [Google Scholar]
- Deng QH, Lu C, Yu YC, Li YG, Sundell J, Norbäck D, 2016b. Early life exposure to traffic-related air pollution and allergic rhinitis in preschool children. Resp. Med 121, 67–43. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R, 1997. Improvement on cross-validation: The 632+ bootstrap method. J, Am. Stat. Assoc 92, 548–560. [Google Scholar]
- Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippilä C, Ahonen S, Nevalainen J, Veijola R, Pekkanen J, Ilonen J, Simell O, Knip M, Virtanen SM, 2009. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin. Exp. Allergy 39, 875–882. [DOI] [PubMed] [Google Scholar]
- Ferrante G, Antona R, Malizia V, Montalbano L, Corsello G, La Grutta S, 2014. Smoke exposure as a risk factor for asthma in childhood: a review of current evidence. Allergy Asthma Proc. 35, 454–461. [DOI] [PubMed] [Google Scholar]
- Godil SS, Parker SL, Zuckerman SL, Mendenhall SK, Devin CJ, Asher AL, McGirt MJ, 2013. Determining the quality and effectiveness of surgical spine care: patient satisfaction is not a valid proxy. Spine J. 13,1006–1012. [DOI] [PubMed] [Google Scholar]
- Guo J, Zhu WJ, Wang HM, Holt PG, Zhang GC, Liu CH, 2019. Risk factors and prognosis of recurrent wheezing in Chinese young children: a prospective cohort study. Allergy Asthma Cl. Im 15: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harving H, Korsgaard J, Dahl R, 1993. House-dust mites and associated environmental conditions in Danish homes. Allergy 48, 106. [DOI] [PubMed] [Google Scholar]
- Hvistendahl M, 2012. Public health. China takes aim at rampant antibiotic resistance. Science, 336, 795. [DOI] [PubMed] [Google Scholar]
- Huang C, Liu W, Hu Y, Zou ZJ, Zhao ZH, Shen L, Weschler LB, Sundell J, 2015. Updated prevalences of asthma, allergy, and airway symptoms, and a systematic review of trends over time for childhood asthma in Shanghai, China. PLoS One 10, e0121577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wang F, Li PF, 2018. Responses of vegetation cover to environmental change in large cities of China. Sustainability 10 (270): doi:10.3390. [Google Scholar]
- Kemp JP, Kemp JA, 2001. Management of Asthma in Children. Am. Fam. Physician 63, 1341–1349. [PubMed] [Google Scholar]
- Kulig M, Luck W, Lau S, Niggemann B, Bergmann R, Klettke U, Guggenmoos-Holzmann I, Wahn U, 1999. Effect of pre and postnatal tobacco smoke exposure on specific sensitization to food and inhalant allergens during the first 3 years of life. Allergy 54, 220–228. [DOI] [PubMed] [Google Scholar]
- Lai CKW, Beasley R, Crane J, Foliaki S, Shah J, Weiland S, the ISAAC Phase Three Study Group, 2009. Global variation in the prevalence and severity of asthma symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 64, 476–483. [DOI] [PubMed] [Google Scholar]
- Lapin BL, Piorkowski J, Owenby D, Wagner-Cassanova C, Freels S, Chavez N, Hernandes E, Pelzel D, Vergara C, Hayes RM, Persky V, 2014. The relationship of early-life antibiotic use with asthma in at-risk children. J Allergy Clin Immunol. 134, 728–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz MD, Quackenboss JJ, 1990. The effects of environmental tobacco on pulmonary fuction, 1990 Indoor air quality, Kasuga H (ed.), Spring-Verlag, Berlin: 147–152. [Google Scholar]
- Lee SY, Chang YS, Cho SH, 2013. Allergic diseases and air pollution. Asia Pac Allergy. 3, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JY, Kwok MK, Leung GM, Schooling CM, 2016. Breastfeeding and childhood hospitalizations for asthma and other wheezing disorders. Ann. Epidemiol 26, 21–27. [DOI] [PubMed] [Google Scholar]
- Li LY, Adamkiewicz G, Zhang YP, Spengler JD, Qu F, Sundell J, 2015. Effect of Traffic Exposure on Sick Building Syndrome Symptoms among Parents/Grandparents of Preschool Children in Beijing, China. PLoS One 10, e0128767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang Q, Shi WJ, Li L, Li Y, Pang Y, Yao B, Jang h., 2013. Epidemiological survey and analysis of asthma in children aged 0-14 years old in urban and rural areas of Chengdu region. Transl. Pediatr 15, 609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR, 1997. Parental History and the Risk for Childhood Asthma Does Mother Confer More Risk than Father? Am. J. Resp. Crit. Care, doi: 10.1164/ajrccm.158.1.9710014 [DOI] [PubMed] [Google Scholar]
- Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR, 2011. Exposure to cockroach allergen in the home is associated with incident doctor-diagnosed asthma and recurrent wheezing. J. Allergy Clin. Immunol 107, 41–47. [DOI] [PubMed] [Google Scholar]
- Liu W, Huang C, Hu Y, Zou ZJ, Sundell J, 2013. Associations between indoor environmental smoke and respiratory symptoms among preschool children in Shanghai, China. Chin. Sci. Bull 58, 4211–4216. [Google Scholar]
- Lodge CJ, Tan DJ, Lau MX, Dai X, Tham R, Lowe AJ, Bowatte G, Allen KJ, Dharmage SC, 2015. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 104, 38–53. [DOI] [PubMed] [Google Scholar]
- Lynch NR, Lopez RI, Di Prisco-Fuenmayor MC, Hagel I, Medouze L, Viana G, Ortega C, Prato G, 1987. Allergic reactivity and socio-economic level in a tropical environment. Clin. Allergy 17, 199–207. [DOI] [PubMed] [Google Scholar]
- Ma Y, Chen YZ, Chen ZL, Cao L, Lin LM, Liu YL, 2002. Epidemiological survey and analysis on children’s asthma in Beijing in 2000 (in Chinese). Beijing Medical Journal, 24, 173–176. [Google Scholar]
- Margolis HG, Mann JK, Lurmann FW, Mortimer KM, Balmes JR, Hammond SK, Tager IB, 2009. Altered pulmonary function in children with asthma associated with highway traffic near residence. Int. J. Environ. Health Res 19, 139–155. [DOI] [PubMed] [Google Scholar]
- Marra F, Marra CA, Richardson K, Lynd LD, Kozyrskyj A, Patrick DM, Bowie WR, Fitzgerald JM, 2009. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 123, 1003–10. [DOI] [PubMed] [Google Scholar]
- Meier L, Van De Geer S and Bühlmann P, 2008. The group lasso for logistic regression. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 70(1), pp.53–71. [Google Scholar]
- Meinsausen N, 2007. Relaxed lasso. Comput. Stat. Data An. 15(1), 374–393 [Google Scholar]
- Miyake Y, Tanaka K, Sasaki S, Kiyohara C, Ohya Y, Fukushima W, Yokoyama T, Hirota Y, the Osaka Maternal and Child Health Study Group, 2008. Breastfeeding and the risk of wheeze and asthma in Japanese infants: The Osaka Maternal and Child Health Study. Pediatr. Allergy Immu 19, 490–496. [DOI] [PubMed] [Google Scholar]
- Naclerio RM, Proud D, Kagey-Sobotka A, Lichtenstein LM, Thompson M, Togias A, 1995. Cold dry air-induced rhinitis: effect of inhalation and exhalation through the nose. J. Appl. Physiol 79, 467–471. [DOI] [PubMed] [Google Scholar]
- Nagano J, Kakuta C, Motomura C, Odajima H, Sudo N, Nishima S, Kubo C, 2010. The parenting attitudes and the stress of mothers predict the asthmatic severity of their children: a prospective study. Biopsychosoc Med. 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cooperative Group on Childhood Asthma (NCGCA), China. 1993. Epidemiolog ical study on bronchial asthma among 900,000 children aged 0-14 years old in China. Chin. J. Tuberculosis Respir. Dis 16(Suppl), 64–83. [Google Scholar]
- Naydenov K, Popov T, Mustakov T, Melikov A, Bornehag CG, Sundell J, 2008. The association of pet keeping at home with symptoms in airways, nose and skin among Bulgarian children. Pediatr. Allergy Immunol 19, 702–708. [DOI] [PubMed] [Google Scholar]
- Nielsen GD, 1991. Mechanisms of activation of the sensory irritant receptor by airborne chemicals. Crit. Rev. Toxicol 21, 183–208. [DOI] [PubMed] [Google Scholar]
- Paaso EMS, Jaakkola MS, Rantala AK, Hugg TT, Jaakkola JJK, 2014. Allergic diseases and asthma in the family predict the persistence and onset-age of asthma: a prospective cohort study. Respiratory Research, dio: 10.1186/s12931-014-0152-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MM, Quinn JW, Jung KH, Hoepner L, Diaz D, Perzanowski M, Rundle A, Kinney PL, Perera FP, Miller RL, 2011. Traffic density and stationary sources of air pollution associated with wheeze, asthma, and immunoglobulin E from birth to age 5 years among New York City children. Environ. Res 111, 1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce N, Aït-Khaled N, Beasley R, Mallol J, Keil U, Mitchell E, Robertson C, the ISAAC Phase Three Study Group, 2007. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood. Thorax 62, 758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D, 2010. Asthma and allergic rhinitis: Linked in treatment and outcomes. Ann. Thorac Med 5, 63–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu F, Weschler LB, Sundell J, Zhang YP, 2013. Increasing prevalence of asthma and allergy in Beijing pre-school children: Is exclusive breastfeeding for more than 6 months protective? Chin. Sci. Bull. 58, 4190–4202. [Google Scholar]
- Risnes KR, Belanger K, Murk W, Bracken MB, 2011. Antibiotic exposure by 6 months and asthma and allergy at 6 years: findings in a cohort of 1,401 US children. Am. J. Epidemiol 173, 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo PM, Sever ML, Zeldin DC, 2009. Indoor allergens in school and daycare environment. J. Allergy Clin. Immunol 124(2): 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone N, Yong HH, Li L, Jiang Y, Fong GT, 2015. Perceived acceptability of female smoking in China: findings from waves 1 to 3 of the ITC China survey. Tob Control. 24, iv48–iv54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon WR, Burge HA, Boise JR, 1980. Exclusion of particulate allergens by window air conditioners. J. Allergy Clin. Immun 65, 305–308. [DOI] [PubMed] [Google Scholar]
- Sun WY, 2017. China’s permanent urbanization rate hits 57.4 percent. People's Daily. http://en.people.cn/n3/2017/0713/c90000-9241304.html [Google Scholar]
- Sun Y, Sundell J, 2011a. Early daycare attendance increase the risk for respiratory infections and asthma of children. J. Asthma 48, 790–796. [DOI] [PubMed] [Google Scholar]
- Sun Y, Sundell J, 2011b. Life style and home environment are associated with racial disparities of asthma and allergy in Northeast Texas children. Sci. Total Environ 409, 4229–4234. [DOI] [PubMed] [Google Scholar]
- Strom-Tejsen P, Weschler CJ, Wargocki P, Myśków D, Zarzycka J, 2008. The influence of ozone on self-evaluation of symptoms in a simulated aircraft cabin. J Expo. Sci. Environ. Epidemiol 18, 272–281. [DOI] [PubMed] [Google Scholar]
- Sundell J, Lindvall T, 1993. Indoor air humidity and sensation of dryness as risk indicators of SBS. Indoor Air 3, 382–390. [Google Scholar]
- Tham KW, Zuraimi MS, Koh D, Chen FT, 2007. Association between home dampness and presence of molds with asthma and allergic symptoms among young children in the tropics. Pediatr. Allergy Immu 18, 418–424. [DOI] [PubMed] [Google Scholar]
- Tibshirani R, 1996. Regression shrinkage and selection via the Lasso. Journal of the Royal Statistical Society. Series B (Methodological), 58, 267–288. [Google Scholar]
- van de Kant KD, Klaassen EMM, Jöbsis Q, Nijhuis AJ, van Schayck OCP, Edward Dompeling E, 2009. Early diagnosis of asthma in young children by using non-invasive biomarkers of airway inflammation and early lung function measurements: study protocol of a case-control study. BMC Public Health. 9, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas PA, Brenner B, Clark S, Boudreaux ED, Camargo CA, 2007. Exposure to environmental tobacco smoke among children presenting to the emergency department with acute asthma: A multicenter study. Pediatr. Pulmonol 42, 646–655. [DOI] [PubMed] [Google Scholar]
- Vastardi M, Katayeva I, Puebla-Neira D, Joks R, 2012. Distance from a heavily trafficked highway is implicated in the presence of allergic rhinoconjunctivitis and asthma in adults. J. Allergy. Clin. Immun 129, AB205. [Google Scholar]
- Wang LF, Qu F, Zhang YP, Weschler LB, Sundell J, 2015. Home environment in relation to allergic rhinitis among preschool children in Beijing, China: A cross-sectional study. Build. Environ 93, 54–63. [Google Scholar]
- Wang J, Li B, Yu W, Wang H, Sundell J, Norbäck D, 2017. Associations between parental health, early life factors and asthma, rhinitis and eczema among-pre-school children in Chongqing, China. Glob. J. Health Sci 9, 121–134. [Google Scholar]
- Wang XY, Liu W, Hu Y, Zou ZJ, Shen L, Huang C, 2016. Home environment, lifestyles behaviors, and rhinitis in childhood. Int. J. Hyg. Environ. Health 219, 220–231. [DOI] [PubMed] [Google Scholar]
- Welch BL, 1947. The generalization of "Student’s" problem when several different population variances are involved. Biometrika. 34 (1-2): 28–35. [DOI] [PubMed] [Google Scholar]
- Wieringa MH, Weyler JJ, Van Bastelaer FJ, Nelen VJ, Van Sprundel MP, Vermeire PA 1997. Higher asthma occurrence in an urban than a suburban area: role of house dust mite skin allergy. Eur Respir J. 10, 1460–1466. [DOI] [PubMed] [Google Scholar]
- Xu A, Xia YR, 2014. The Changes in Mainland Chinese Families During the Social Transition: A Critical Analysis. J. Comparative Family Studies 45, 31–53. [Google Scholar]
- Xu XF, Li YJ, Sheng YJ, Liu JL, Tang LF, Cheng ZM, 2014. Effect of low birth weight on childhood asthma: a meta-analysis. BMC Pediatr. 14, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Rao Y, Wang L, Liu S, Guo JJ, Sharma M, et al. , 2017. Smoking in pregnancy: a cross-sectional study in China. Tob Induc Dis. doi: 10.1186/s12971-017-0140-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Chiu J, Chiu H, Kao W, 1997. Damp housing conditions and respiratory symptoms in primary school children. Pediatr. Pulm 24, 73–77. [DOI] [PubMed] [Google Scholar]
- Yuan M, Lin Y, 2006. Model selection and estimation in regression with grouped variables. J. Roy. Stat. Soc.: Series B (Statistical Methodology), 68, 49–67. [Google Scholar]
- Zhang KH, 2017. Urbanization and Industrial Development in China. Springer; Singapore. [Google Scholar]
- Zhang YP, Li BZ, Huang C, Yang X, Qian H, Deng QH, Zhao ZH, Li AG, Zhao JN, Zhang X, Qu F, Hu Y, Yang J, Zhang M, 2013a. Ten cities cross-sectional questionnaire survey of children asthma and other allergies in China. Chin. Sci. Bull 58, 4182–4189. [Google Scholar]
- Zhang YP, Mo JH, Weschler CJ, 2013b. Reducing health risks from indoor exposures in today’s rapidly developing urban China, Environ. Health Persp 121, 751–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YM, Zhang J, Liu SL, Zhang X, Yang SN, Gao J, Zhao J, Chen H, Chen XX, Sun FX, Shen L, Wang DY, 2013c. Prevalence and associated risk factors of allergic rhinitis in preschool children in Beijing. Laryngoscope 123, 28–35. [DOI] [PubMed] [Google Scholar]
- Zhao J, Bai JA, Shen KL, Xiang L, Huang S, Chen AH, Huang Y, Wang JS, Ye RW, 2010. Self-reported prevalence of childhood allergic diseases in three cities of China: a multicenter study. BMC Public Health 10, 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, 2006. The adaptive lasso and its oracle properties. J. Am. Statist. Assoc, 101, 1418–1429. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.