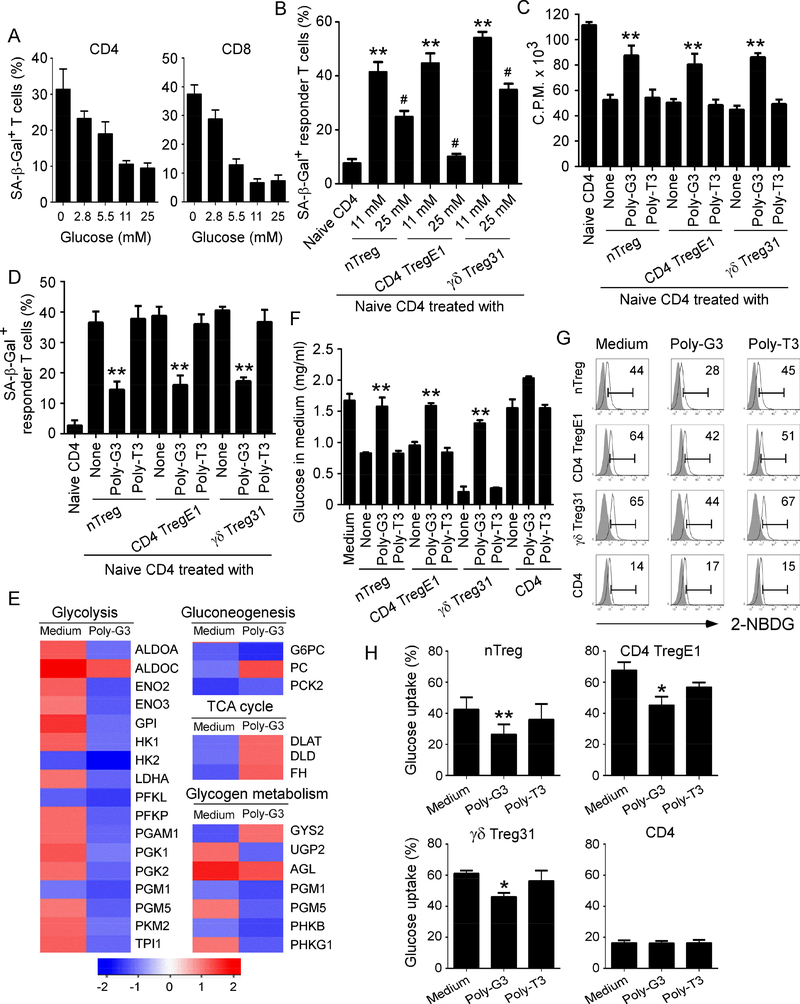
Figure 3. TLR8 signaling suppresses glucose uptake and metabolism in human Treg cells.
(A) Significantly increased SA-β-Gal+ T cell populations were induced in anti-CD3-activated naïve CD4+ and CD8+ T cells cultured in the medium with different concentrations of glucose for 3 days. Data shown are mean ± SD of T cells from three individual healthy donors. Normal medium with 11 mM glucose served as a control. (B) Addition of high concentration of glucose markedly rescued responder T cell senescence induced by nTreg cells and tumor-derived Treg cells. Anti-CD3 activated CD4+ T cells were co-cultured with Treg cells for 3 days with different concentrations of glucose. SA-β-Gal expression in responder CD4+ T cells was determined. Data shown are mean ± SD from three independent experiments. **p<0.01, compared with the naïve CD4 only group. #p<0.01, compared with the Tregtreated with normal concentration of glucose (11mM) group. (C) and (D) TLR8 ligand Poly-G3 treatment significantly reversed Treg suppressive capacity on T cell proliferation (in C) and prevented Treg-induced responder T cell senescence (in D). Different types of Treg cells were co-cultured with naïve CD4+ T cells in the presence or absence of Poly-G3 (3 μg/ml) or Poly-T3 (control) for 3 days. Proliferation of cocultured naïve T cells stimulated by anti-CD3 antibody was determined by [3H]-thymidine incorporation assays (in C), and SA-β-Gal expression in treated naïve T cells was determined (in D). Data shown are mean ± SD from representative of three independent experiments with similar results. **p<0.01, compared with the respective medium only and Poly-T3 treatment groups. (E) Alterations of genes involved in glucose metabolism were identified and ranked in nTreg cells after treatment with Poly-G3 at 24 hours. Gene alterations were normalized to log2 expression level. Human nTreg cells were isolated from PBMCs of two healthy donors and treated with Poly-G3 for different time points. Total RNA was purified and pooled, and transcriptome analyses of Treg cells were performed using the Illumina wholegenome Human HT-12 BeadChips. (F) Poly-G3 treatment increased glucose levels in the culture medium of both nTreg and tumor-derived Treg cells. Different types of Treg cells and naïve CD4+ T cells were cultured in the presence or absence of Poly-G3 or Poly-T3 (Control) for 3 days, and glucose levels in the culture medium were determined. **p<0.01, compared with the None and Poly-T3 treatment groups. (G) and (H) Poly-G3 treatment significantly decreased glucose uptake by nTreg and tumorderived Treg cells. Glucose uptake was determined by the flow cytometry with addition of 2-NBDG for 15 min after 3 day culture. Results shown in histogram (H) are mean ± SD from four independent experiments. *p<0.05 and **p<0.01, compared with the respective medium only and control Poly-T3 treatment groups.