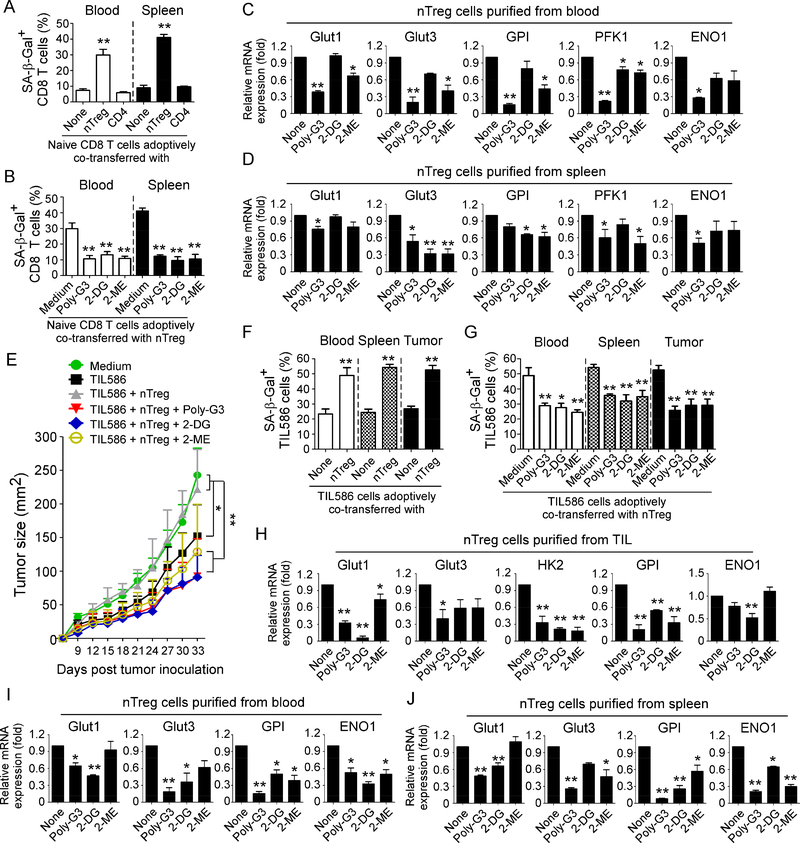
Figure 7. Reprogramming of glucose metabolism in Treg cells via TLR8 signaling activation or specific metabolic inhibition enhances anti-tumor immunity and tumor immunotherapy in vivo.
(A) Increased senescent cell populations were markedly induced in pre-activated CD8+ T cells after cotransfer with nTreg cells but not with control effector CD4+ T cells. Naïve CD8+ T cells (5 × 106/mouse), expanded nTreg (3 × 106/mouse) and CD4+CD25− T cells (3 × 106/mouse) were pre-activated with antiCD3 antibody and adoptively co-transferred into NSG mice. Blood and Spleens were harvested at 12 days post-injection. The transferred human CD8+ T cells were isolated for subsequent SA-β-Gal staining. **p<0.01, compared with the groups co-transferred with CD4+CD25− T cells or CD8+ T cells alone. (B) Treatment with Poly-G3, 2-DG, or 2-ME significantly prevented induction of senescence in transferred CD8+ T cells. nTreg cells were pretreated with Poly-G3 (3 μg/mL), glycolytic metabolism inhibitor 2-DG (1 mM) or HIF1α inhibitor 2-ME (10 μM) for 24 hours prior to adoptively transfer into the mice. After adoptive transfer of T cells into the mice, Poly-G3 (50 μg/mouse), 2-DG (10 mg /mouse), or 2-ME (0.3 mg /mouse) were intraperitoneally injected into mice for a total of 3 doses with 3-day intervals. Cell preparation and injection procedures were the same as in (A). The transferred human CD8+ T cells in different organs were isolated at 12 days post-injection for subsequent SA-β-Gal staining. **p<0.01, compared with the medium group. (C) and (D) Real-time PCR quantification of expression changes of glucose transporters and glycolytic enzymes in purified nTreg cells from blood and spleens of NSG mice treated with Ploy-G3 and different inhibitors. Cell treatment and adoptive transfer procedure were identical to (B). The transferred nTreg cells were isolated for RT-PCR analyses.*p<0.05 and **p<0.01, compared with the co-transferred group without inhibitor treatment. (E) to (G) Treatments with Poly-G3 and inhibitors prevented tumor-specific T cell senescence and enhanced antitumor immunity in NSG mice. Human 586mel tumor cells (5 × 106/mouse) were subcutaneously injected into NSG mice. Tumorspecific CD8+ TIL586 cells (5 × 106/mouse) were i.v. injected on day 5 with or without nTreg cells (3 × 106/mouse). In addition, nTreg cells were pretreated with TLR8 ligand Poly-G3, glycolytic metabolism inhibitor 2-DG and HIF1α inhibitor 2-ME for 24 hours prior to adoptively transfer into the mice, and mice were then intraperitoneally injected with Poly-G3, 2-DG or 2-ME for a total of 3 doses with 3-day intervals after T cell transfer. The treatment procedures and doses were identical to the experiments in (B). Tumor volumes were measured and presented as mean ± SD (in E) (n=5 mice per group). Blood, spleens, and tumors were harvested at day 33 post injection. The transferred human TIL586 T cells in different organs were isolated for SA-β-Gal staining. *p<0.05 and **p<0.01, compared between the comparison groups (in E), or compared with the groups of TIL586 alone (in F) or co-transferred group without inhibitor treatment (in G), respectively. (H) to (J) Real-time PCR quantification of glucose transporter and glycolytic enzyme expression changes in purified Treg cells from TILs, blood and spleens of tumor-bearing NSG mice treated with Ploy-G3 and different inhibitors. Cell treatment and adoptive transfer procedure were identical to (E). The transferred nTreg cells were isolated from different organs and tumor tissues for RT-PCR analyses.*p<0.05 and **p<0.01, compared with the co-transferred group without inhibitor treatment.