FIGURE 1.
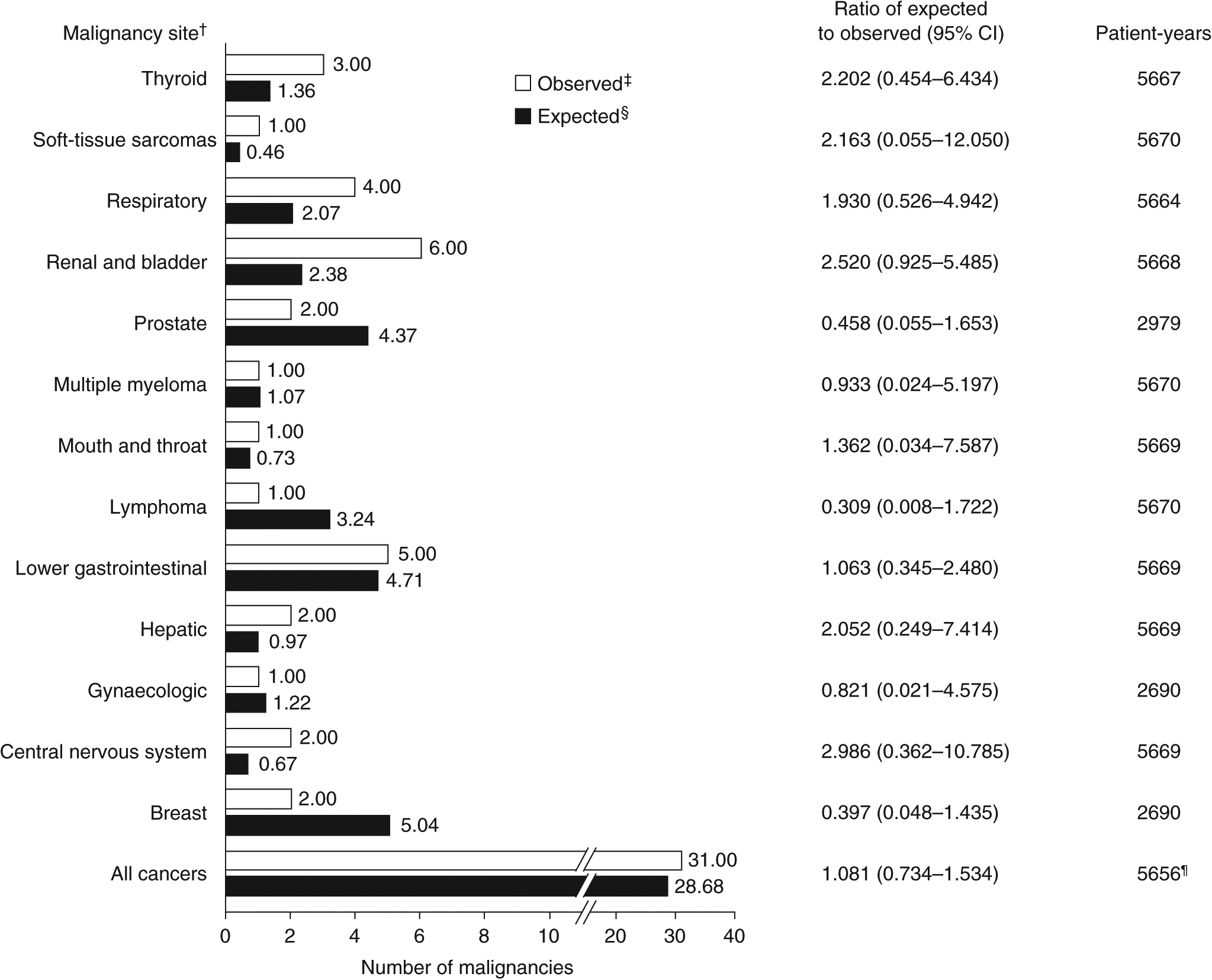
Indirect standardisation of the numbers of GEMINI LTS study patients with malignancy events by anatomical site. Data shown exclude malignancies with a diagnosis reported prior to, or within 1 y after starting treatment with vedolizumab and the following events: benign neoplasms, basal cell carcinomas, colon adenomas, haemangioma, neurilemmoma, non-melanoma skin cancers and malignancies with diagnoses reported before vedolizumab initiation. CI, confidence interval; LTS, long-term safety. †Malignancies were identified using Medical Dictionary for Regulatory Activities version 20.0 System Organ Class term “neoplasms benign, malignant, and unspecified (including cysts and polyps)”. Benign neoplasms, basal cell carcinoma, colon adenoma, haemangioma, neurilemmoma, all non-melanoma skin cancers and malignancies with diagnoses reported before, or within 1 y after, the start of vedolizumab treatment (ie those in patients who received placebo in the lead-in studies) were excluded. The corresponding follow-up time for excluded malignancies was not used in the analysis.‡Patients with more than one malignancy at a single site were counted only once. §The expected number of patients with malignancies in the GEMINI LTS study was estimated by indirectly standardising against age- and sex-specific malignancy rates in patients with inflammatory bowel disease in Optum’s Clinformatics Data Mart database. ¶Survival time was measured up to time of failure or end of study follow-up. As a result, the total number of patient-years is reduced vs values of each site individually
