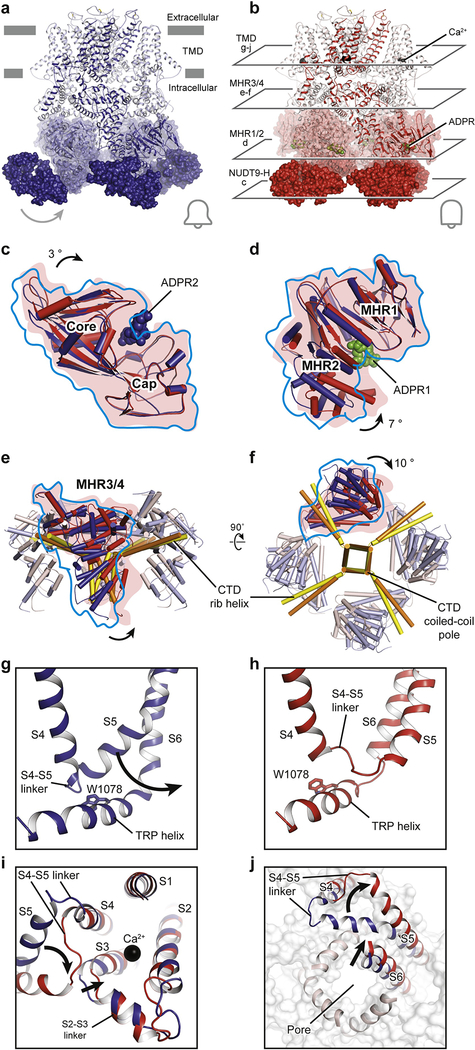
Figure 5: Gating mechanism of the voltage-independent TRPM2 channel.
Structures of the (a) EDTA-drTRPM2 and (b) ADPR/Ca2+-drTRPM2. Comparison of the (c) NUDT9-H domains and the (d) MHR1/2 domains of EDTA-hsTRPM2 and ADPR/Ca2+-hsTRPM2 by superimposition of the cap regions or the MHR1 domains. ADPR1 and ADPR2 are shown as green and blue spheres. The domain closure induced by ADPR binding in NUDT9-H and MHR1/2 is indicated. (e, f), Superimposition of the linker layers of EDTA-hsTRPM2 (blue) and ADPR/Ca2+-hsTRPM2 (red) by aligning the CTD coiled-coil poles. (g, h), Detailed view of the interaction between MHR4 and the TRP helix. The movement of the S4-S5 linker is indicated and the Trp1078 of TRP helix is shown in sticks. (i, j), Superimposition of EDTA-drTRPM2 (blue) and ADPR/Ca2+-drTRPM2 (red) structures by aligning the S1-S4 domains. (i), The conformational changes of S2-S3 linker and S3 upon Ca2+ binding are indicated. (j), The flipping of S4-S5 linker upon channel opening, viewed from the intracellular side, is indicated.