Figure 2.
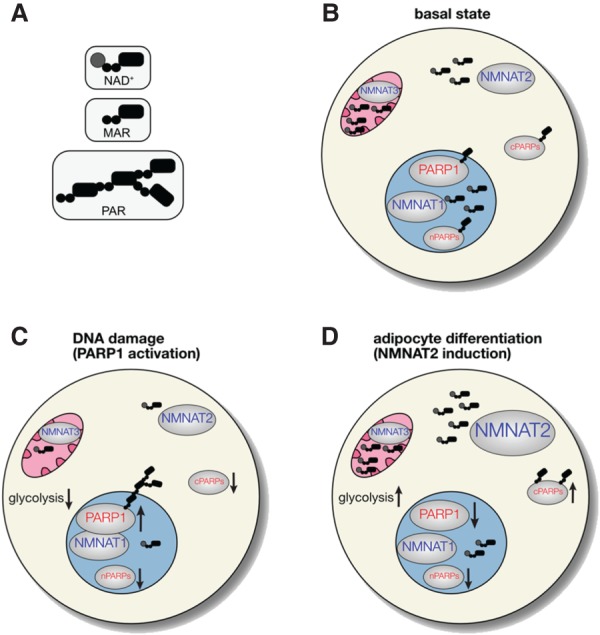
NAD+ synthesis is compartmentalized in mammalian cells and regulates—and is regulated by—the NAD+ consumer PARP1. NMNAT1–3, which synthesize NAD+, are distinctly localized in major subcellular compartments: NMNAT1 in the nucleus, NMNAT2 in the cytoplasm, and NMNAT3 in mitochondria. (cPARPs) Cytoplasmic PARPs; (nPARPs) nuclear PARPs. (A) Cartoon representation of NAD+, mono-ADP-ribose (MAR), and poly-ADP-ribose (PAR). (B) Under basal conditions, the concentration of NAD+ is highest in mitochondria (∼250 μM), followed by the cytoplasm and nucleus (both ∼100 μM). The number of NAD+ molecules indicates relative subcellular concentrations of NAD+. (C) During DNA damage, PARP1 associates with NMNAT1 and is activated (up arrow), leading to auto-poly-ADP-ribosylation (PARylation). This results in a temporal reduction of NAD+ levels in the nucleus, and likely the cytoplasm and mitochondria. The decrease in NAD+ levels in all compartments is expected to decrease the activity of nPARPs and cPARPs, as well as glycolytic flux and oxidative phosphorylation (down arrows). (D) Upon adipocyte differentiation, NMNAT2 is induced, which increases NAD+ levels in the cytoplasm and leads to an increase in glycolytic flux and perhaps the activity of cPARPs (up arrows). The increase in NMNAT2 levels depletes NMN from the nucleus (not shown), leading to lower NAD+ levels in the nucleus and a decrease in the activity of PARP1 and perhaps other nPARPs (down arrows).
