Embedded in the diagnosis of Idiopathic Pulmonary Fibrosis (IPF) is the long-standing belief that this progressive fibrotic lung condition arises spontaneously and its cause is unknown 1. While the exceptional work of Nureki and colleagues 2 chips away at this fundamental concept, these investigators have also created a translational model that may accelerate drug discovery for a disease that often results in death within 3-5 years 1.
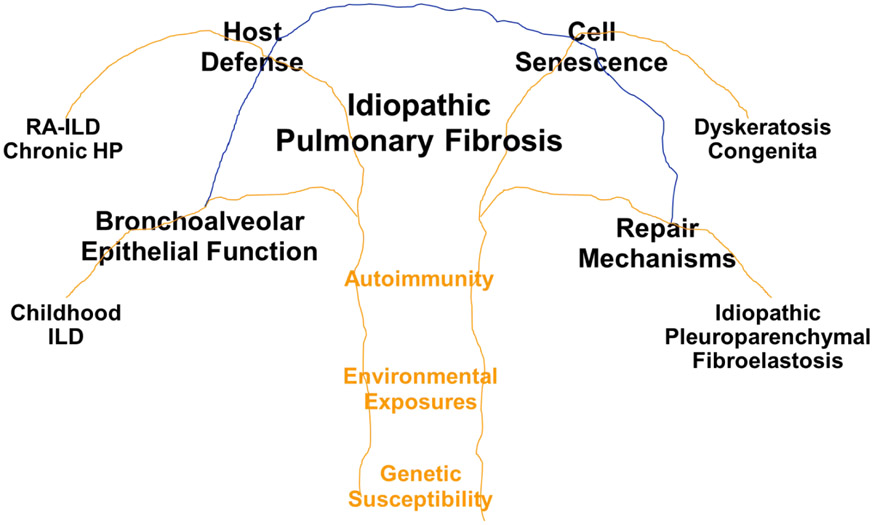
IPF is a complex phenotype, typified by clinical, etiologic, and molecular heterogeneity. In fact, the radiographic and pathological features of usual interstitial pneumonia (UIP) require heterogeneity to definitively diagnose IPF 1. Gene variants (rare and common sequence variants in 7 genes [MUC5B, TERT, TERC, RTEL1, PARN, SFTPC, and SFTPA2; 3-11] and in at least 12 novel loci 12,13), environmental exposures (asbestos and microorganisms), and immune conditions (rheumatoid arthritis and scleroderma;14) place individuals at risk of developing the radiographic and pathological features of UIP. The more common risk factors, such as age, male sex, cigarette smoking, and the MUC5B promoter variant predispose individuals to develop phenocopies of IPF 3, including rheumatoid arthritis associated interstitial lung disease (RA-ILD) 15 and chronic hypersensitivity pneumonitis 16. The heterogeneity of IPF is highlighted further by the multiple emerging epigenetic 17 and transcriptional 18-20 profiles reported in this disease. The collective clinical, etiologic, and molecular heterogeneity of IPF suggests that this disease represents a nonspecific response to recurrent environmental and endogenous injury in a susceptible host that is unable to resolve the progressive fibrotic response due to defects in one or several key mechanisms involved in lung homeostasis. Consequently, integrating key etiologic attributes (genetic susceptibility, environmental exposures, and autoimmunity) with known mechanisms of disease, such as host defense, bronchoalveolar cell function, cell senescence, and lung repair could establish a roadmap for more effective treatment of early and established disease (Figure 1).
Figure 1.
IPF is a heterogeneous disease, caused by abnormalities in host defense, bronchoalveolar cell function, cell senescence, and lung repair, all of which are affected by genetic variants, environmental exposures, and autoimmunity. While each of these biological processes can fail substantially and result in unique types of lung fibrosis (ChILD, RA-ILD, chronic HP, dyskeratosis congenital, or idiopathic pleuroparenchymal fibroelastosis), it is likely that most cases of IPF occur in those with mild to modest defects in one or several of these key mechanisms of lung homeostasis.
To understand further the mechanisms that induce pulmonary fibrosis, Nureki and colleagues 2 focused on surfactant protein C gene (SFTPC) -associated pulmonary fibrosis. While SFTPC mutations are unusual in patients with IPF, they are more often observed in children with interstitial lung disease (ChILD) or families with early onset of interstitial lung disease 9,21-27. Nevertheless, understanding how mutations in SFTPC cause pulmonary fibrosis could more generally advance our understanding of the etiology and pathogenesis of IPF. Nureki pursued this research by introducing a missense substitution (1286T>C) of the surfactant protein C gene (SFTPCI73T) into mice and regulating the expression of this mutant gene in type II alveolar epithelia (AT2). This gain-of-function rare variant involves the C-terminal or BRICHOS domain of proSP-C, and is the most common SFTPC mutation in humans with SFTPC-associated ILD. Their results demonstrate that this single missense substitution results in the spontaneous development of lung fibrosis, presumably caused by altered intracellular trafficking of surfactant protein C proprotein, defective proteostasis, impaired mitophagy, and enhanced macroautophagy (but not apoptosis) of AT2 cells. These molecular events were associated with the spontaneous development of acute alveolitis with overexpression of IPF biomarkers (SP-D, MMP-7, OPN, and IL-6), and fibrotic remodeling, including hyperplasia of AT2 cells but no specific features of UIP. Hence, Nureki and colleagues 2 have demonstrated the potential role of surfactant protein C and AT2 cells in the development of pulmonary fibrosis, and by pursuing functional genomic strategies, these investigators have developed a spontaneous model of interstitial lung disease that may prove useful as a translational platform for IPF.
Surfactant protein C is a hydrophobic peptide with a number of biologic functions related to its ability to lower surface tension at low lung volumes, including facilitating the spreading and adsorption of the phospholipids comprising surfactant at the air/liquid interface, variably inserting into the phospholipid monolayer as a function of surface tension, facilitating re-spreading of surfactant following alveolar compression at low volumes, and promoting surfactant recycling. Numerous investigators have noted that alveolar collapse and collapse induration are involved in the pathogenesis of pulmonary fibrosis 28, and both alveolar collapse and collapse induration will increase in the absence of normally functioning surfactant or impaired AT2 cell production or recycling of surfactant. The chronic endoplasmic reticulum stress resulting from cyclical airspace opening and closing of atelectatic alveoli, and/or from over-distension of alveoli adjacent to areas of collapse induration may explain the peripheral, heterogeneous distribution, and the progression of the fibrotic lesions in IPF.
The intriguing work of Nureki and colleagues 2 has solidified some of the basic concepts in pulmonary fibrosis, created a translational model of fibrotic lung disease, and reinforced the importance of genetic targets in understanding the etiology and pathogenesis of this disease. More specifically, their research has reinforced the importance of AT2 cell injury in the initial stages of pulmonary fibrosis. As emphasized in the associated editorial 29, their research also demonstrates that endogenous defects in cellular function, as well as those induced by environmental exposures, can cause recurrent microscopic injury to the alveolar space, and poorly functioning AT2 cells can serve as initiating, and perhaps recurrent events in the fibroproliferative process. Although no murine model has yet recapitulated the complex heterogeneity and pathogenesis of IPF, the spontaneous model of SFTPC-associated ILD created by Nureki and colleagues 2 will undoubtedly prove valuable in understanding the biology of pulmonary fibrosis and developing novel drugs for this progressive disease.
Acknowledgements
The authors thank Drs. Peter Henson, Joyce Lee, Marvin Schwartz, and Ivana Yang for their critique of this manuscript and thoughtful advice. The preparation of this manuscript was supported by the National Heart, Lung and Blood Institute (UH2/3-HL123442, R01-HL097163, R21/R33-HL120770, and P01-HL092870) and Department of Defense (W81XWH-17-1-0597).
Footnotes
Competing Interests
D.A.S. is the founder and chief scientific officer of Eleven P15, a company focused on the early diagnosis and treatment of pulmonary fibrosis. D.A.S. has patents awarded (US Patent no: 8,673,565) and submitted (US Patent application no: 62/250,390, US Patent application no: 62/525,087, and US Patent application no: 62/525,088) for the treatment and diagnosis of fibrotic lung disease. D.A.S. serves on the scientific advisory boards of Apellis Pharmaceuticals, NuMedii, and Pliant Therapeutics, and has consulted for Arrowhead Pharmaceuticals and Pieris Pharmaceuticals.
References
- 1.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44–e68. [DOI] [PubMed] [Google Scholar]
- 2.Nureki SI, Tomer Y, Venosa A, et al. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J Clin Invest 2018;128:4008–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 2011;364:1503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 2007;356:1317–26. [DOI] [PubMed] [Google Scholar]
- 5.Tsakiri KD, Cronkhite JT, Kuan PJ, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A 2007;104:7552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas AQ, Lane K, Phillips J 3rd, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med 2002;165:1322–8. [DOI] [PubMed] [Google Scholar]
- 7.Lawson WE, Grant SW, Ambrosini V, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax 2004;59:977–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Kuan PJ, Xing C, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet 2009;84:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Moorsel CH, van Oosterhout MF, Barlo NP, et al. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a dutch cohort. Am J Respir Crit Care Med 2010;182:1419–25. [DOI] [PubMed] [Google Scholar]
- 10.Cogan JD, Kropski JA, Zhao M, et al. Rare Variants in RTEL1 Are Associated with Familial Interstitial Pneumonia. Am J Respir Crit Care Med 2015;191:646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuart BD, Choi J, Zaidi S, et al. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat Genet 2015;47:512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fingerlin TE, Murphy E, Zhang W, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet 2013;45:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fingerlin TE, Zhang W, IV Y, et al. Genome-wide Imputation Study Identifies Novel HLA Locus for Pulmonary Fibrosis and Potential Role for Auto-Immunity in Idiopathic Interstitial Pneumonia (IIP). In Review 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010;35:1322–8. [DOI] [PubMed] [Google Scholar]
- 15.Juge PA, Lee JS, Ebstein E, et al. MUC5B Promoter Variant rs35705950 is a Risk Factor for Rheumatoid Arthritis - Interstitial Lung Disease. New Engl J Med 2018;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ley B, Newton CA, Arnould I, et al. The MUC5B promoter polymorphism and telomere length in patients with chronic hypersensitivity pneumonitis: an observational cohort-control study. The Lancet Respiratory medicine 2017;5:639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang IV, Pedersen BS, Rabinovich E, et al. Relationship of DNA methylation and gene expression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2014;190:1263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selman M, Pardo A, Barrera L, et al. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med 2006;173:188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminski N Microarray analysis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:S32–6. [PubMed] [Google Scholar]
- 20.Yang IV, Coldren CD, Leach SM, et al. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax 2013;68:1114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nogee LM, Dunbar AE 3rd, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med 2001;344:573–9. [DOI] [PubMed] [Google Scholar]
- 22.Thomas AQ, Lane K, Phillips J 3rd, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med 2002;165:1322–8. [DOI] [PubMed] [Google Scholar]
- 23.Cameron HS, Somaschini M, Carrera P, et al. A common mutation in the surfactant protein C gene associated with lung disease. J Pediatr 2005;146:370–5. [DOI] [PubMed] [Google Scholar]
- 24.Brasch F, Griese M, Tredano M, et al. Interstitial lung disease in a baby with a de novo mutation in the SFTPC gene. Eur Respir J 2004;24:30–9. [DOI] [PubMed] [Google Scholar]
- 25.Abou Taam R, Jaubert F, Emond S, et al. Familial interstitial disease with I73T mutation: A mid- and long-term study. Pediatric pulmonology 2009;44:167–75. [DOI] [PubMed] [Google Scholar]
- 26.Crossno PF, Polosukhin VV, Blackwell TS, et al. Identification of early interstitial lung disease in an individual with genetic variations in ABCA3 and SFTPC. Chest 2010;137:969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beers MF, Hawkins A, Maguire JA, et al. A nonaggregating surfactant protein C mutant is misdirected to early endosomes and disrupts phospholipid recycling. Traffic (Copenhagen, Denmark) 2011;12:1196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burkhardt A Alveolitis and collapse in the pathogenesis of pulmonary fibrosis. Am Rev Respir Dis 1989;140:513–24. [DOI] [PubMed] [Google Scholar]
- 29.Blackwell TS. Lung injury and fibrosis induced by a mutant form of surfactant protein C. J Clin Invest 2018;128:3745–6. [DOI] [PMC free article] [PubMed] [Google Scholar]