Figure 1.
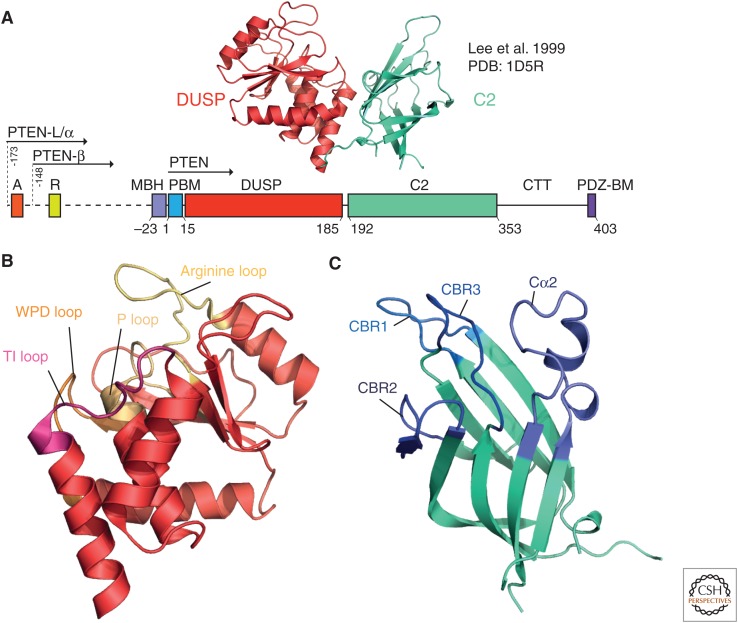
PTEN crystal structures and variants. (A) Domain organization of PTEN and translational variants. The crystal structure (Lee et al. 1999) is shown above in cartoon-format, colored by domain, with the dual-specificity phosphatase (DUSP) domain in red and the C2 domain in teal. The domain organization of PTEN consists of the PIP2-binding motif (PBM), DUSP, and C2 domains, followed by the carboxy-terminal tail (CTT) and the PDZ-binding motif. Translational variants of PTEN show amino-terminal extensions, with PTEN-L/α having a 173-amino-acid disordered extension, and PTEN-β having a 148-amino-acid extension. The amino-terminal extension contains a polyalanine (domain “A,” 12–17-L) and polyarginine (“R,” 47–52-L), as well as a membrane-binding α-helix ([MBH], 151–174-L). (B) The DUSP domain. The three core loops of the active site of PTEN (the TI, WPD, and P loops) are shown, along with the arginine loop, key for membrane binding. (C) The calcium-independent C2 domain of PTEN, with the calcium-binding region (CBR) loops highlighted, along with the enlarged Cα2 loop.