Abstract
Background
The significance of endoscopic activity in asymptomatic ulcerative colitis (UC) patients with an ileal pouch is unknown.
Aim
We aimed to understand the association of endoscopic pouch activity in asymptomatic patients with the development of pouchitis.
Methods
We analyzed a retrospective cohort of patients with UC or IBD-Unspecified (IBD-U) who underwent a total proctocolectomy with ileal pouch anal anastomosis (IPAA). Asymptomatic patients with a Pouchitis Disease Activity Index (PDAI) symptom sub-score of zero who underwent an index surveillance pouchoscopy were included. Endoscopic pouch body activity was graded as 0: normal, 1: mucosal inflammation, or 2: mucosal breaks (ulcers and/or erosions). The primary outcome was primary acute idiopathic pouchitis defined as PDAI score ≥ 7 with symptoms lasting less than four weeks and responsive to standard antibiotics, not otherwise meeting criteria for secondary pouchitis. The secondary outcome was chronic idiopathic pouchitis defined as PDAI score ≥ 7 with symptoms lasting greater than four weeks despite standard antibiotics. Predictors of pouchitis were analyzed using Kaplan-Meier and Cox regression methods with hazard ratios (HR) and 95% confidence intervals (CI) reported.
Results
A total of 143 asymptomatic pouch patients were included. Index endoscopic pouch body activity was graded as 0 in 86 (60.1%) patients, 1 in 26 (18.2%) patients and 2 in 31 (21.7%) patients. The median length of follow-up after index surveillance pouchoscopy was 3.03 [IQR 1.24–4.60] years. Primary acute idiopathic pouchitis occurred in 44 (31%) patients and chronic idiopathic pouchitis in 12 (8.4%) patients. Grade 2 endoscopic pouch activity was associated with the development of acute pouchitis (HR 2.39, 95% CI 1.23–4.67), however not chronic pouchitis (HR 1.76, 95% CI 0.53–5.87). Histologic inflammation in endoscopically normal pouch mucosa was not associated with acute or chronic pouchitis.
Conclusions
Mucosal breaks are present in nearly a quarter of asymptomatic pouch patients and are associated with an increased risk of acute pouchitis.
Keywords: Ulcerative colitis, colectomy, ileal pouch anal anastomosis, pouchitis
INTRODUCTION
Total proctocolectomy (TPC) with ileal pouch anal anastomosis (IPAA) is the standard surgical procedure for ulcerative colitis (UC) patients with medically refractory disease or dysplasia.1 Pouchitis is the most common inflammatory condition in pouch patients with cumulative incidence rates of 25% at one year, 35% at three years, and 45% at five years.2 Approximately 60% of patients develop at least one recurrence of pouchitis and up to 20% develop chronic pouchitis.3
There are limited published data on the correlation of endoscopic disease activity with symptoms in pouch patients. Two previous studies have observed that approximately 25% of pouch patients with symptoms of pouchitis have no underlying endoscopic or histologic evidence of disease, while approximately 45% of asymptomatic pouch patients have abnormal endoscopic and histologic findings.4,5 There are no available data on the clinical outcomes of asymptomatic pouch patients with underlying endoscopic disease activity. The clinical significance of these incidental endoscopic findings is unknown.
In recent years, inflammatory bowel disease (IBD) treatment guidelines have adopted a “treat to target” strategy with a composite target of clinical remission and endoscopic healing.6 This composite outcome includes subjective patient symptoms and objective endoscopic data, the latter of which are particularly important since symptoms may not reliably correlate with endoscopic disease activity.7 Endoscopic healing in UC and Crohn’s disease (CD) has been associated with improved outcomes such as sustained long-term clinical remission and decreased risk of disease progression.8,9 The “treat to target” strategy has not been applied to pouch patients due to lack of data regarding the predictive value of endoscopic activity on pouch outcomes.
Surveillance pouchoscopy is frequently performed in asymptomatic pouch patients after final surgical stage at our institution. We therefore aimed to study the association of endoscopic activity in asymptomatic pouch patients with the development of primary acute idiopathic and chronic idiopathic pouchitis.
METHODS
Study Population
We performed a retrospective analysis of a cohort of pouch patients from a large tertiary care IBD center. All patients with a history of UC or IBD-unspecified (IBD-U) who underwent TPC with IPAA at the Mount Sinai Hospital for medically refractory disease or dysplasia between January 2008 and December 2017 were identified through hospital electronic medical records. Only asymptomatic patients with a Pouchitis Disease Activity Index (PDAI) symptom sub-score of zero and an initial post-IPAA pouchoscopy performed strictly for surveillance were included.10 Included patients did not have any documented episodes of pouchitis nor pouchitis treatments prior to index surveillance pouchoscopy.
Patients less than 18 years of age at the time of colectomy and those with subsequent development of secondary pouchitis were excluded. Secondary pouchitis was defined as pouch inflammation related to pelvic sepsis, ischemia, autoimmune conditions, or infection.3 Pelvic sepsis was defined by Mount Sinai Hospital surgeons as an infective process in the peri-pouch area that resulted in unstable vital signs or re-exploration without an abscess or leak identified. Ischemia was defined endoscopically by asymmetric distribution of inflammation present at the distal half to quarter of the pouch or in one limb of the pouch with sharp demarcation between inflamed and uninflamed areas.11 Autoimmune pouchitis was defined as active mucosal inflammation in the setting of positive serum anti-nuclear antibody, IgG4, and/or anti-microsomal antibody.3 Infectious pouchitis was defined as pouchitis secondary to pathogens identified on stool culture or polymerase chain reaction.3
Data Collection and Variables
Clinical information was abstracted from the medical record using a standardized data collection sheet by five investigators (MK, MR, AR, MP, ET). Study data were collected and managed using REDCap electronic data capture tools hosted at Mount Sinai Hospital.12 All collected data were reviewed by a single investigator (MK) who was blinded to outcomes. Collected patient demographics and disease characteristics included sex, age at colectomy, disease duration, body mass index (BMI) at colectomy, preoperative diagnosis (UC or IBD-U), extra-intestinal manifestations specifically primary sclerosing cholangitis (PSC) or arthritis, family history of IBD, or the presence of backwash ileitis on colectomy specimen. Collected surgical characteristics included indication, procedures and stages, and type of anastomosis (handsewn or stapled). Collected pouchoscopy details included procedure indications, endoscopic findings, histological diagnoses, and post-procedure medication changes.10 PDAI endoscopic and symptom sub-scores were calculated at the time of chart review based on documented symptoms and pouchoscopy findings.
We assessed endoscopic pouch body activity utilizing criteria established by the PDAI endoscopic sub-score. Endoscopic activity in the pouch body was graded as 0: normal, 1: mucosal inflammation with mucosal edema, granularity, friability, loss of vascular pattern, mucosal exudate without mucosal breaks, or 2: any number of mucosal breaks (ulcers, erosions). Ulcers along staple lines were excluded from the endoscopic grade. Histologic activity was defined as any microscopic inflammation reported as pouchitis or ileitis on official clinical pathology report in any pouch body biopsy.
Outcomes
Primary idiopathic pouchitis was defined as the following: (1) total PDAI score ≥ 7 with symptoms of increased stool frequency from postoperative baseline, rectal bleeding, fecal urgency, abdominal cramps, or fever (temperature ≥ 37.8), (2) abnormal pouchoscopy with mucosal edema, granularity, friability, loss of vascular pattern, mucosal exudate, and/or ulceration and histologic inflammation, and (3) absence of secondary causes of pouchitis such as pelvic sepsis, ischemia, autoimmune conditions, or infection as defined above. Clinical and endoscopic components of the outcome were assessed within one week of each other. Primary idiopathic pouchitis was further classified as either acute or chronic. Acute pouchitis was defined as symptoms lasting less than four weeks and responding to two-week courses of antibiotics.13 Chronic pouchitis was defined as symptoms lasting greater than four weeks despite standard antibiotic courses and requiring chronic antibiotic or anti-inflammatory therapy.13
Statistical Analysis
Descriptive statistics were performed to describe baseline characteristics of the study population and are reported as proportions or medians (with interquartile range, IQR) for categorical and continuous variables, respectively. Cox regression and Kaplan-Meier methods were used to compare the risk of clinical pouch outcomes between patients with and without endoscopic or histologic activity in the pouch body. Hazard ratios (HR) and 95% confidence intervals (CI) are reported. Independent variables that were associated with pouchitis and significant at the p ≤ 0.1 level in univariable analysis were incorporated into multivariable models. All analyses were performed using SAS v9.4 (SAS Institute, Cary, NC). Two-sided p values < 0.05 were considered statistically significant.
RESULTS
Pouchoscopy data were available for 386 patients who underwent TPC with IPAA between January 2008 and December 2017. Of these, 143 patients were asymptomatic with a PDAI symptom sub-score of zero and underwent pouchoscopy a median of 412 [IQR 225–701] days after IPAA completion for surveillance at the discretion of their surgeon or gastroenterologist. Patient and surgical characteristics are given in Table 1. The majority of patients were male (55.9%) and had a preoperative diagnosis of UC (94.4%). Of note, no patients with IBD-U subsequently developed Crohn’s disease-like condition. The median age at colectomy was 38 [IQR 28–53] years and median pre-colectomy disease duration was 84 [IQR 30–162] months. Colectomy indication was medically refractory disease in 105 (73.4%) patients and dysplasia in 38 (26.6%) patients.
Table 1.
Clinical characteristics of asymptomatic pouch patients undergoing surveillance pouchoscopy, total n=143 for all variables
| Characteristics | n (%) |
|---|---|
| Sex | |
| ● Male | 80 (55.9%) |
| ● Female | 63 (44.1%) |
| Age at colectomy, years* | 38 [28–53] |
| Disease duration, months* | 84 [30–162] |
| BMI at colectomy, kg/m2 * | 23.4 [20.1–26.7] |
| Preoperative diagnosis | |
| ● Ulcerative colitis | 135 (94.4%) |
| ● IBD-U | 8 (6.6%) |
| Preoperative extent of disease | |
| ● Extensive colitis | 117 (81.3%) |
| ● Left sided colitis | 27 (18.8%) |
| Extraintestinal manifestations | |
| ● PSC | 6 (4.2%) |
| ● Arthritis | 10 (7.0%) |
| Tobacco use at colectomy | 6 (4.2%) |
| Backwash ileitis | 20 (14.0%) |
| Surgical indication# | |
| ● Medically refractory disease | 105 (73.4%) |
| ● Dysplasia | 38 (26.6%) |
BMI: body mass index, IBD-U: IBD-unspecified, PSC: primary sclerosing cholangitis
median [interquartile range]
Of the 143 asymptomatic patients who underwent index surveillance pouchoscopy, endoscopic pouch body activity was graded as 0 in 86 (60.1%) patients, 1 in 26 (18.2%) patients, and 2 in 31 (21.7%) patients. Pouch body biopsies were taken from 81 patients total, specifically from 31/86 (36.0%) patients with grade 0 pouch body activity, 19/26 (73.1%) patients with grade 1, and all patients with grade 2. Histologic inflammation was noted in 24/31 (77.4%) patients with grade 0 pouch body activity, 16/19 (84.2%) patients with grade 1, and 26/31 (83.9%) patients with grade 2 (Figure 1). The median length of follow-up after index surveillance pouchoscopy was 3.03 [IQR 1.24–4.60] years.
Figure 1.
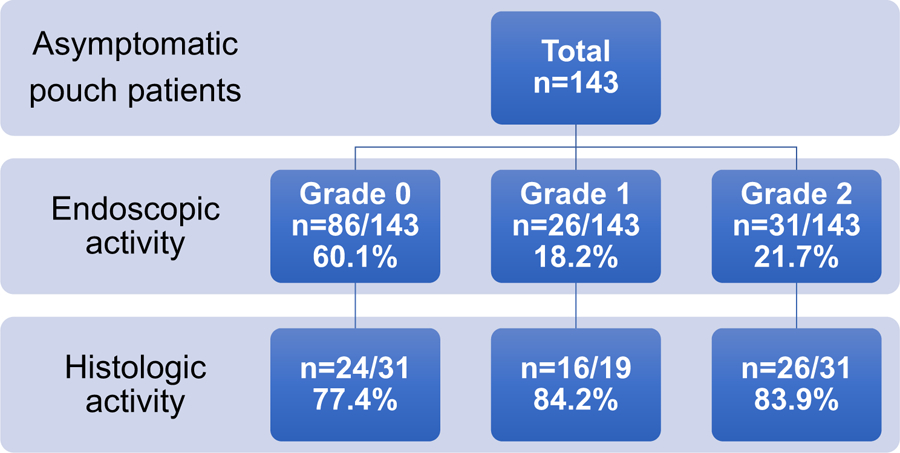
Distribution of endoscopic and histologic pouch body activity in asymptomatic patients.
Endoscopic activity grade 0: normal, grade 1: mucosal inflammation with erythema, edema, granularity, and/or friability without mucosal breaks, grade 2: any number of mucosal breaks with ulcers and/or erosions.
Biopsies were taken from the pouch body in 81 patients in total, specifically 31 with grade 0 endoscopic activity, 19 with grade 1, and 31 with grade 2.
Primary acute idiopathic pouchitis occurred in 44/143 (31.0%) patients a median of 1.9 [IQR 1.0–3.9] years after index surveillance pouchoscopy. Primary acute idiopathic pouchitis developed in 12/44 (27.3%) patients within the first year after index pouchoscopy, 16/44 (36.4%) patients within the second year, 8/44 (18.2%) patients within the third year, and 8/44 (18.2%) within years four through six. Chronic idiopathic pouchitis occurred in 12/143 (8.4%) patients a median of 2.7 [IQR 1.1–4.4] years after index surveillance pouchoscopy. No cases of secondary pouchitis were identified or excluded.
Grade 2 endoscopic pouch activity was associated with the development of primary acute idiopathic pouchitis (HR 2.39, 95% CI 1.23–4.67) (Figure 2), however not chronic pouchitis (HR 1.76, 95% CI 0.53–5.87). The sensitivity and specificity of grade 2 endoscopic activity to predict subsequent primary acute idiopathic pouchitis were 29.6% and 81.8%, respectively. The positive and negative predictive values of grade 2 endoscopic activity for subsequent primary acute idiopathic pouchitis were 41.9% and 72.3%, respectively. Grade 1 endoscopic pouch activity was not associated with the development of acute pouchitis (HR 1.05, 95% CI 0.45–2.44). Histologic activity in patients with normal, grade 0 endoscopic activity was not associated with the development of acute pouchitis (HR 0.57, 95% CI 0.20–1.60).
Figure 2.
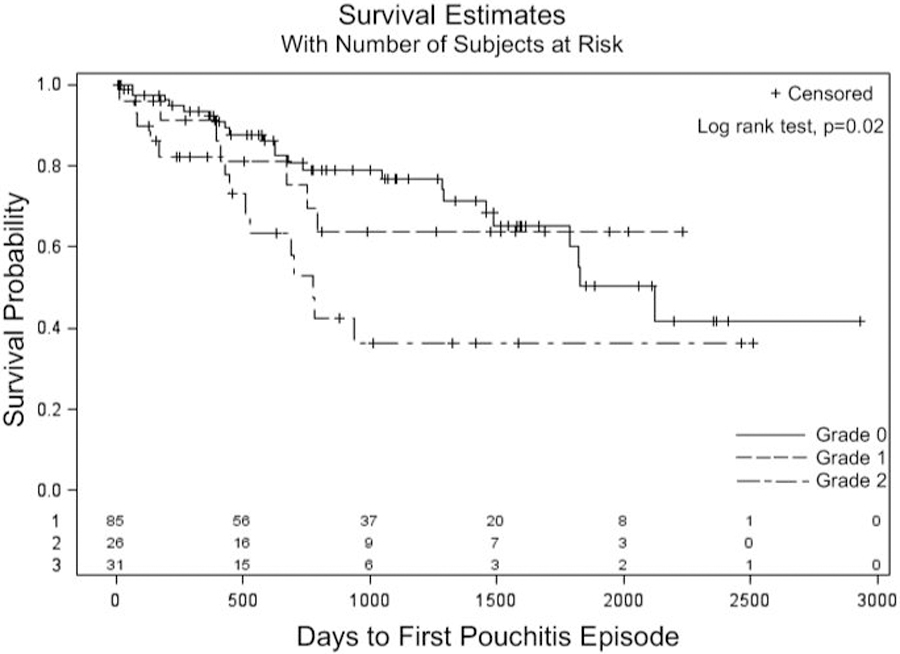
Kaplan-Meier estimates of developing acute pouchitis after index surveillance pouchoscopy in asymptomatic patients with grade 0, 1 or 2 endoscopic pouch activity. Endoscopic activity grade 0: normal, grade 1: mucosal inflammation with erythema, edema, granularity, and/or friability without mucosal breaks, grade 2: any number of mucosal breaks with ulcers and/or erosions.
The only baseline covariable significantly associated with the development of acute pouchitis was PSC (HR 3.8, 95% CI 1.34–10.74) (Table 2). Of note, acute pouchitis developed in 13/44 (29.5%) patients with grade 2 endoscopic activity, 4/6 (66.7%) patients with PSC, and 3/3 (100%) patients with both PSC and grade 2 endoscopic activity. Antibiotics were prescribed to 4/26 (15.4%) patients with grade 1 endoscopic activity and 16/31 (51.6%) patients with grade 2 endoscopic activity immediately after surveillance pouchoscopy at the discretion of their surgeon or gastroenterologist. On multivariable analysis, controlling for PSC and antibiotic use, grade 2 endoscopic activity remained significantly associated with the development of acute pouchitis (adjusted HR 2.1, 95% CI 1.08–4.04).
Table 2.
Cox Regression: Univariable Predictors of Future Acute Pouchitis
| Co-Variable | HR (95% CI) | p value |
|---|---|---|
| Disease duration | 1.00 (0.997–1.003) | 0.99 |
| Interval time between surgery and pouchoscopy | 1.00 (0.99–1.00) | 0.53 |
| BMI at colectomy, kg/m2 | 0.99 (0.94–1.06) | 0.97 |
| Age at first scope | 0.99 (0.97–1.01) | 0.34 |
| Male sex (reference = female) | 1.50 (0.81–2.81) | 0.20 |
| Extensive colitis (reference = left-sided colitis) | 1.91 (0.75–4.84) | 0.17 |
| Tobacco use at colectomy | 0.54 (0.08–3.95) | 0.55 |
| Primary sclerosing cholangitis | 3.80 (1.34–10.74) | 0.01 |
| Endoscopic activity grade 1 (reference = grade 0) | 1.05 (0.45–2.44) | 0.92 |
| Endoscopic activity grade 2 (reference = grade 0) | 2.39 (1.23–4.67) | 0.01 |
BMI: body mass index
Endoscopic activity (treated as categorical variable): grade 0: normal, grade 1: mucosal inflammation with erythema, edema, granularity, and/or friability without mucosal breaks, grade 2: any number of mucosal breaks with ulcers and/or erosions
DISCUSSION
We observed that mucosal erosion or ulceration was present in nearly a quarter of asymptomatic pouch patients and was associated with the development of primary acute idiopathic pouchitis. Previous studies have examined the frequency of incidentally noted pouch disorders on surveillance pouchoscopy, however our study is the first to report an association between endoscopic pouch body activity in asymptomatic patients and future acute pouchitis.5
There are no standardized guidelines for the surveillance of pouch patients. The European Crohn’s and Colitis Organization (ECCO) and American Society for Gastrointestinal Endoscopy (ASGE) are the only professional societies that recommend annual pouchoscopy in patients with such risk factors as PSC, dysplasia or colorectal cancer before or at the time of colectomy, and/or pouch mucosa with persistent atrophy or severe inflammation.14–16 No specific pouch surveillance protocol is recommended for the majority of pouch patients who are asymptomatic and without risk factors, and studies from the United States and Europe indicate significant heterogeneity in practice patterns and pouchoscopy surveillance intervals.17,18
Much has been published about the importance of endoscopic healing in UC and CD patients on long-term clinical outcomes; however, to our knowledge, there are no data on the predictive value of endoscopic activity on pouch outcomes or applicable “treat to target” recommendations. In our study, the association between mucosal breaks and acute pouchitis persisted after controlling for antibiotic use. This suggests that mucosal healing and suppression of mucosal breaks is a reasonable target that could mitigate the risk of future pouchitis. It may not be necessary, however, to achieve deep histologic healing given its lack of association with pouchitis. Based on these data, prospective studies should investigate a “treat to target” strategy focused on the resolution of mucosal breaks and the subsequent risk of acute pouchitis.
Our study had several strengths. First, all patients underwent surgery and clinical follow-up at one institution and all charts were systematically reviewed by one investigator blinded to outcomes. Second, our study had a large sample size relative to previously published data and included long-term follow-up. Third, all outcomes of interest were defined strictly according to clinical and endoscopic criteria of the PDAI. Our study also had a number of limitations. First, the study was retrospective and thus subject to selection bias. Given that our cohort is from a single center these results may not be broadly generalizable; however, the majority of IPAA surgeries occur at tertiary care centers similar to our study location.19 Second, although surveillance pouchoscopy is frequently performed at our institution in asymptomatic pouch patients, not all patients underwent a baseline pouchoscopy. This is due to a lack of a standardized surveillance protocol and the fact that the majority of our patients did not have PSC or pre-colectomy dysplasia that would otherwise warrant surveillance pouchoscopy. As a result, actual rates of endoscopic activity and subsequent pouchitis may be different than what was observed. Third, we restricted our study to asymptomatic patients without a history of pouchitis and calculated PDAI scores based on chart review and pouchoscopy reports. Given the retrospective nature of our cohort, there is the chance that limitations in routine chart documentation may have resulted in the inclusion of some patients who did have clinical pouchitis before baseline pouchoscopy. Fourth, while we tried to exclude cases of secondary pouchitis related to pelvic sepsis, ischemia, autoimmune conditions, and infection based on chart review, not all patients had documented serum or stool studies performed to assess these causes. As such, we cannot completely exclude all cases of secondary pouchitis. However, we think this is unlikely to significantly confound our results given the overall rare occurrence of secondary pouchitis.3,11
In conclusion, baseline mucosal breaks in asymptomatic pouch patients are associated with the development of future primary acute idiopathic pouchitis. A “treat to target” strategy applied to the pouch with suppression of mucosal ulcers or erosions may be enough to suppress the risk of pouchitis. Previous studies have reported endoscopic remission with the use of biologic agents for the management of chronic antibiotic refractory pouchitis, however the true definition of endoscopic remission is not well established.20–24 Prospective studies are needed to confirm our observations, delineate the meaning of endoscopic remission in the pouch, and assess the feasibility of achieving endoscopic remission in the pouch.
Acknowledgements
Funding
RCU is supported by a Career Development Award from the Crohn’s and Colitis Foundation and an NIH K23 Career Development Award (K23KD111995–01A1). RPH is supported by a Career Development Award from the Crohn’s and Colitis Foundation.
RPH has served as an advisory board member for Janssen and Takeda and has received research funding from Intralytix. BC has served as an advisory board member or consultant for Abbvie, Alfasigma, Celltrion, Ferring, Grifols, Janssen, and Sublimity Therapeutics. JFC has served as a consultant for Abbbie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Landos, Ipsen, Medimmune, Merck, Novartis, Pfizer, Shire, Takeda, Tigenix and has research grants from Abbvie, Janssen and Takeda. MCD has served as a consultant for Janssen, Takeda, Pfizer, Celgene, and Abbvie and has research grants from Pfizer and Abbvie. RCU has served as an advisory board member or consultant for Janssen, Pfizer and Takeda and has research grants from Abbvie, Boehringer Ingelheim, and Pfizer.
Abbreviations
- UC
ulcerative colitis
- TPC
total protocolectomy
- IPAA
ileal pouch anal anastomosis
- IBD-U
inflammatory bowel disease unspecified
- PSC
primary sclerosing cholangitis
- BMI
body mass index
Footnotes
Conflict of Interest
The remaining authors have no relevant conflicts of interest or disclosures.
REFERENCES
- 1.Ross H, Steele SR, Varma M, et al. Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the surgical treatment of ulcerative colitis. Dis Colon Rectum. 2014;57:5–22. [DOI] [PubMed] [Google Scholar]
- 2.Ferrante M, Declerck S, De Hertogh G, et al. Outcome after proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Inflamm Bowel Dis. 2008;14:20–28. [DOI] [PubMed] [Google Scholar]
- 3.Shen B. Acute and chronic pouchitis – pathogenesis, diagnosis and treatment. Nat Rev Gastroenterol Hepatol. 2012;17;9(6):323–33. [DOI] [PubMed] [Google Scholar]
- 4.Shen B, Achkar JP, Lashner BA, et al. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology. 2001;121:261–7. [DOI] [PubMed] [Google Scholar]
- 5.Zhu H, Wu XR, Queener E, et al. Clinical value of surveillance pouchoscopy in asymptomatic ileal pouch patients with underlying inflammatory bowel disease. Surg Endosc. 2013;27(11):4325–32. [DOI] [PubMed] [Google Scholar]
- 6.Peyrin-Biroulet L, Sandborn W, Sands B, et al. Selecting therapeutic targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324–38 [DOI] [PubMed] [Google Scholar]
- 7.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–95. [DOI] [PubMed] [Google Scholar]
- 8.Shah SC, Colombel JF, Sands BE, et al. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14(9):1245–1255. [DOI] [PubMed] [Google Scholar]
- 9.Shah SC, Colombel JF, Sands BE, et al. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther. 2016;43(3):317–33. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ, Tremaine WJ, Batts KP, et al. Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin Proc. 1994;69:409–415. [DOI] [PubMed] [Google Scholar]
- 11.Quinn KP, Lightner AL, Faubion WA, et al. A Comprehensive Approach to Pouch Disorders. Inflamm Bowel Dis. 2019;25(3):460–471. [DOI] [PubMed] [Google Scholar]
- 12.Harris P, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazio V, Kiran R, Remzi F, et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013;257(4):679–85. [DOI] [PubMed] [Google Scholar]
- 14.Annese V, Beaugerie L, Egan L, et al. European evidence-based consensus: inflammatory bowel disease and malignancies. J Crohns Colitis 2015;9:945–65. [DOI] [PubMed] [Google Scholar]
- 15.Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extraintestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11(6):649–670. [DOI] [PubMed] [Google Scholar]
- 16.Shergill AK, Lightdale JR, Bruining DH, et al. ; American Society for Gastrointestinal Endoscopy Standards of Practice C. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc 2015;81:1101–21; e1–13. [DOI] [PubMed] [Google Scholar]
- 17.Gu J, Remzi FH, Lian L, Shen B. Practice pattern of ileal pouch surveillance in academic medical centers in the United States. Gastroenterol Rep. 2016;4:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samaan MA, Forsyth K, Segal JP, et al. Current practices in ileal pouch surveillance for patients with ulcerative colitis: a multinational, retrospective cohort study. J Crohns Colitis. 2018. December 24. doi: 10.1093/ecco-jcc/jjy225. [DOI] [PubMed] [Google Scholar]
- 19.Hoang C, Davids K, Wyman A et al. A steady trend but a general re-distribution of elective IPAA for ulcerative colitis In: The American Society of Colon and Rectal Surgeons Annual Meeting Abstracts; May 19–23, 2018; Nashville, Tennessee: Abstract S34. [Google Scholar]
- 20.Ferrante M, D’Haens G, Dewit O, et al. ; Belgian IBD Research Group. Efficacy of infliximab in refractory pouchitis and Crohn’s disease-related complications of the pouch: a belgian case series. Inflamm Bowel Dis. 2010;16:243–9. [DOI] [PubMed] [Google Scholar]
- 21.Bar F, Kuhbacher T, Dietrich NA, et al. Vedolizumab in the treatment of chronic, antibiotic dependent or refractory pouchitis. Aliment Pharmacol Ther. 2018;47(5):581–587. [DOI] [PubMed] [Google Scholar]
- 22.Weaver KN, Gregory M, Syal G, et al. Ustekinumab is effective for the treatment of Crohn’s disease of the pouch in a multicenter cohort. Inflamm Bowel Dis. 2019; 25(4):767–774. [DOI] [PubMed] [Google Scholar]
- 23.Gregory M, Weaver KN, Hoversten P, et al. Efficacy of vedolizumab for refractory pouchitis of the ileo-anal pouch: results from a multicenter US cohort. Inflamm Bowel Dis. 2019. February 27. [DOI] [PMC free article] [PubMed]
- 24.Segal JP, Ding NS, Worley G, et al. Systematic review with meta-analysis: the management of chronic refractory pouchitis with an evidence-based treatment algorithm. Aliment Pharmacol Ther. 2017; 45(5):581–592. [DOI] [PubMed] [Google Scholar]


