Figure 6.
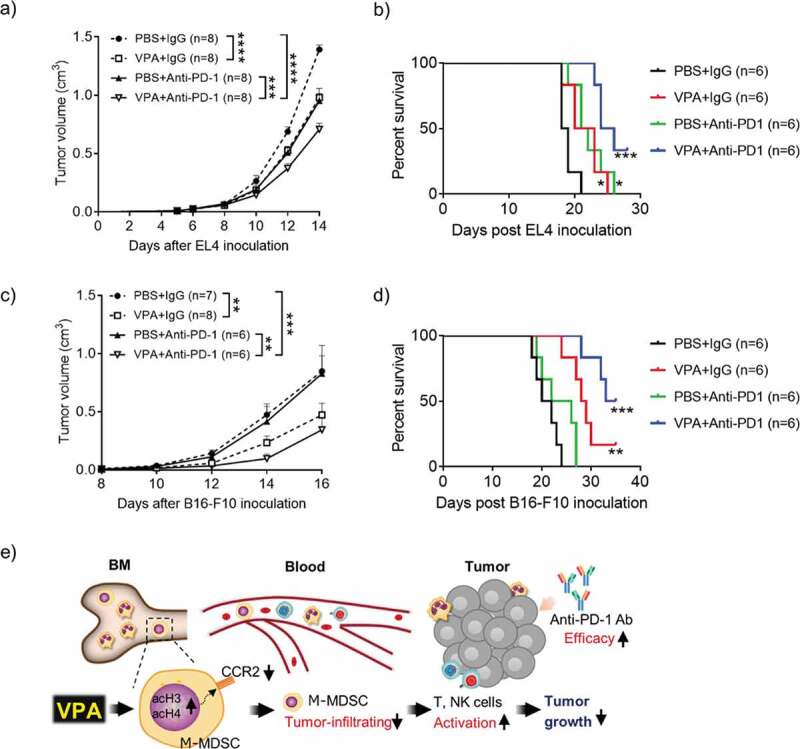
VPA enhances response to anti-PD-1 immunotherapy.
(a, b) EL4 tumor-bearing mice were treated daily with PBS or VPA (500 mg/kg) from day 5 to day 14 in combination with an anti-PD-1 Ab or IgG (400 µg/mouse) on days 5, 8, and 11. (c, d) B16-F10 tumor-bearing mice were treated daily with PBS or VPA (500 mg/kg) from day 8 to day 16, in combination with an anti-PD-1 Ab or IgG (400 µg/mouse) on days 8, 11, and 14. (a, c) Tumor volumes were calculated periodically and are shown as means ± S.E.M., pooled from two independent experiments with n = 6–8 (**p < .01, ***p < .001, and ****p < .0001 by two-way ANOVA) (b, d) Survival of EL4 and B16-F10 tumor-bearing mice (n = 6 per group) was examined. (*p < .05, **p < .01, ***p < .001 compared with PBS + IgG group by Log-rank test). (e) VPA down-regulated CCR2 expression on M-MDSCs via HDAC modification and reduced M-MDSCs migration into tumor site, resulting in CD8 and NK activation, leading to reduction of tumor as illustrated. Our findings show that VPA in combination with an immunotherapeutic agent could be a potential new anti-cancer therapy.
