Abstract
PURPOSE
Vietnam is undergoing rapid socio-economic transition with an increasing cancer burden. The contribution of modifiable risk factors to cancers in Vietnam has not been studied. Therefore, we sought to evaluate the attributable causes of cancer in Vietnam.
METHODS
We reviewed the data on burden of cancer in Vietnam from 2 cancer registries in Hanoi and Ho Chi Minh City between 1995 and 2012. Next, we calculated the fractions of cancers occurring in 2018 attributable to established modifiable risk factors whose impact could be quantified. Data on exposure prevalence were obtained for the period from 2000 to 2010 from national sources wherever possible.
RESULTS
Cancer incidence in Vietnam has decreased slightly in both sexes. Cancer related to infectious agents decreased sharply, whereas cancer related to nutrition and metabolism has increased. In 2018, established carcinogens included in the analysis explained 47.0% of cancer burden in Vietnam. Chronic infections accounted for 29.1% of cancers (34.7% in men and 22.1% in women), tobacco smoking for 13.5% (23.9% in men and 0.8% in women), and alcohol drinking for 10.3%. Passive smoking was responsible for 8.8% of cancers in women. Other risk factors, including overweight or obesity, nulliparity, and low vegetable and fruit intake, accounted for < 1% of all cancers each.
CONCLUSION
Cancer incidence is slowly decreasing in Vietnam, and the causes of more than half of cancers remain unexplained. This result underlines the need for further epidemiologic and fundamental research. Our findings confirm the notion that controlling oncogenic infections and decreasing tobacco smoking are the most effective approaches to reduce the burden of cancer in Vietnam, but other risk factors, including alcohol drinking and diet, should not be neglected.
INTRODUCTION
Vietnam has undergone rapid and far-reaching economic development during the past decades. Over the past 30 years, average growth of the gross national product has been 6.7% per year, one of the highest in the world.1 In parallel, the country has experienced impressive progress toward improving the health status of the population; estimated life expectancy in Vietnam in 2018 was 76.3 years (71.7 years for men and 80.9 years for women),2 a level that is higher than that in many countries with similar levels of gross domestic product per capita. As in other less developed countries that underwent a rapid socioeconomic and health transition, the increase in life expectancy was primarily a result of a decline in mortality from communicable and maternal and childhood diseases. Consequently, noncommunicable diseases, and cancer in particular, have increased their contribution to the burden of disease.3
Although there have been several efforts to evaluate the role of modifiable risks factors for cancer in Vietnam,4-7 systematic study on such issues is unavailable. Under this evolving scenario, we aim to provide a comprehensive assessment of the burden of cancer and the role of its main causes in Vietnam today by calculating population attributable fractions (AFs).
METHODS
We reviewed the data on the burden of cancer in Vietnam. Next, we calculated the fractions of cancers in Vietnam in 2018 attributable to known modifiable causes of cancer for which sufficient data were available.
Burden of Cancer: Sources of Data
The Cancer Incidence in Five Continents program compiles data from cancer registries around the world that satisfy a set of quality criteria, including the proportion of microscopically verified cancers, the proportion of cancers identified from death certificates, and the proportion of patients of unknown age, allowing for valid comparisons across areas and time periods. Updates of data are published every 5 years; the most recent collection (Volume XI) comprises data for 2008-2012.8 The only registry from Vietnam, which was included in Volume XI of the series, is that of Ho Chi Minh City (HCMC), which was also included in Volume VIII (data from 1993-1997).9 Volume VIII also included data from the Cancer Registry of Hanoi.
CONTEXT
Key Objective
What are the attributable causes of cancer in Vietnam?
Knowledge Generated
Almost half of all cancer cases in Vietnam in 2018 are attributable to known modifiable risk factors and therefore can be prevented, at least in theory. Infectious agents, tobacco (including passive smoke) and alcohol consumption are leading causes for cancer in Vietnam.
Relevance
This result underlines the need for further epidemiological and fundamental research. Controlling oncogenic infections and tobacco smoking are the most effective approach to reduce the burden of cancer in Vietnam, but other risk factors, including alcohol drinking and diet, should not be neglected.
In addition, the GLOBOCAN program provides estimates of cancer incidence, mortality, and prevalence from the whole country. The most recent version of GLOBOCAN provides data for 2018.10 Briefly, the 2018 estimates for cancer incidence in Vietnam are based on weighted average from the most recent data from the HCMC Cancer Registry with 2018 population. Mortality data were estimated from incidence estimates, using mortality/incidence ratios derived from cancer registries in neighboring countries. Prevalence data were estimated using national incidence data and prevalence ratios from Nordic countries for the period of 2000-2009 and scaled using Human Development Index (HDI) ratios.11,12
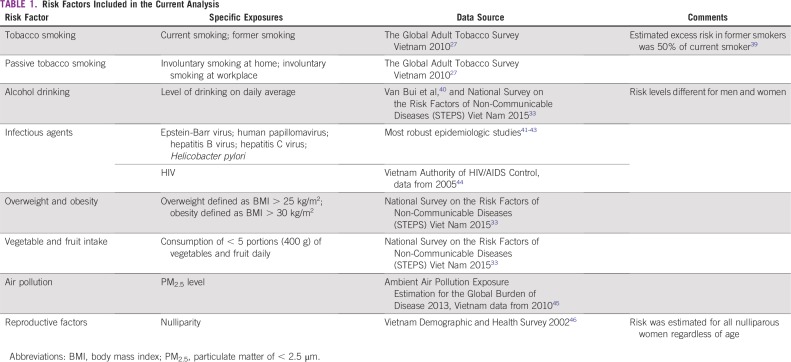
Risk Factors Included in the Current Analysis
Risk factors for each cancer type were included in the estimate of AFs if they were classified either as established human carcinogens (Group 1) by the International Agency for Research on Cancer (IARC)13 or as agents with convincing or probable evidence by the World Cancer Research Fund (WCRF).14 The main risk factors included in the analysis were infectious agents, tobacco smoking, alcohol drinking, passive smoking, pollutants (particulate matter < 2.5 μm [PM2.5]), overweight and obesity, nulliparity, and low vegetable and fruit intake. Although it has never been evaluated by either IARC or WCRF, nulliparity, as a reproductive factor, was included in the review because a large body of evidence supports its role in breast cancer.15,16 Other reproductive factors linked to cancer in humans were not included as a result of insufficient exposure data in Vietnam. Some established carcinogens were not included in the analysis because exposure is rare (eg, radioiodine, radium and its decay products) or because data on the prevalence of such exposures were unavailable (eg, exposure to occupational carcinogens, exogenous hormones, x-ray radiation, γ-radiation, or UV light). Genetic factors for cancer were also excluded because they are unavoidable. The risk factors retained in the analysis are listed in Table 1.
TABLE 1.
Risk Factors Included in the Current Analysis
Risk Factors: Exposure Prevalence
We obtained the sex-specific prevalence of exposure to the risk factors from census data or representative nation-scaled surveys. Where nationally representative data were unavailable, we obtained data on prevalence of exposure from the most robust epidemiologic studies conducted in Vietnam. Data sources are listed in Table 1. The cancer burden observed at any given time reflects past exposures’ cumulative effect. The time between the relevant exposure and the development of cancer (commonly defined as latency) varies by cancer and risk factor and is not always well defined. For most associations between cancers and risk factors, we chose a latency of 10-15 years between exposure and cancer onset (ie, we considered exposures occurring around 2000-2010), if relevant exposure data were available.
Relative Risks
Because of the unavailability of robust studies to determine the association between carcinogens and cancers in Vietnam by the time we conducted this analysis, we obtained relative risks (RRs) from recent published meta-analyses, with preference given to studies in Asia. If meta-analyses were unavailable, results from pooled analyses, cohort studies, and case-control were considered in the respective order. If > 1 RR for the same risk factor was available, preference was given to the study with largest sample size and with results most comprehensively adjusted for confounders. Where there was a statistically significant difference between RRs among males and females, sex-specific RRs were extracted. Otherwise, total RRs were applied for both sexes.
AF Calculation
AF estimates the proportion of cancer that could be avoided if exposure to a given agent were eliminated. We used the AF formula proposed by Levin,17 which is widely used by epidemiologists worldwide, to calculate the AF of an association between a dichotomous exposure and a cancer:
where PF1 is the underlying prevalence of the risk factor in the population and RR is the RR of developing a type of cancer when exposed to the risk factor.
For polytomous exposures, we used the formula described by Hanley18 to calculate the total AF for that exposure:
which can be extended to more than 2 exposure categories.
For some infections, AFs were obtained from generally accepted results from published studies because no data for RR were available.19,20 This applied to AFs for Epstein-Barr virus (EBV) for nasopharyngeal cancer and Hodgkin lymphoma, Helicobacter pylori for stomach cancer, and HIV for Kaposi sarcoma and non-Hodgkin lymphoma.
Combining AFs
For each type of cancer, the individual AFs for different risk factors cannot be numerically added together because a patient may have been exposed to more than one of them. With insufficient information on the nature of risk factors’ interactions and their combined distributions, to estimate the total AFs, we assumed that there was independence of both exposure prevalence and carcinogenic action between risk factors and applied the following formula:
RESULTS
Burden of Cancer
The overall incidence of cancer (age-standardized rates, excluding nonmelanoma skin cancer) in HCMC during 2008-2012 was 130.1 per 100,000 in men and 105.8 per 100,000 in women. The incidence for 1995-1998 was 143.1 per 100,000 in men and 108.6 per 100,000 in women, equivalent to an average decline in cancer incidence of 0.7% in men and 0.2% in women.
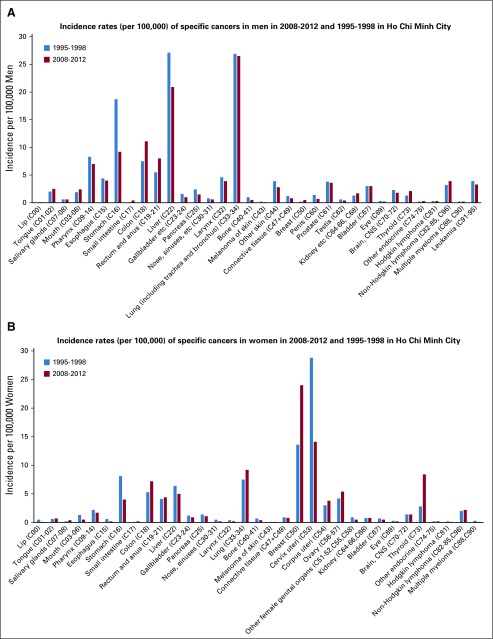
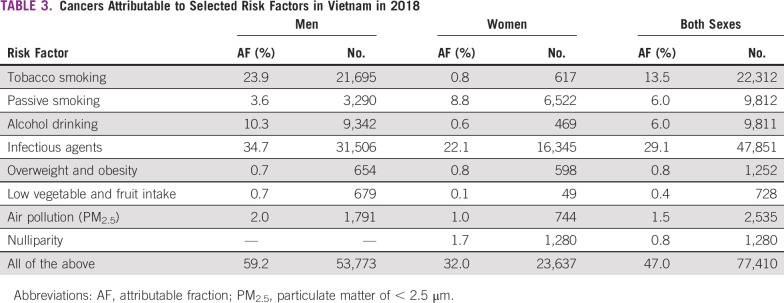
Figures 1A and 1B report incidence rates of specific cancers in 2008-2012 and 1995-1998 in HCMC by sex. Among men, the 3 most common cancers are lung, liver, and stomach cancer; the incidence of the 2 latter cancers decreased remarkably during the 2 time periods, whereas that of lung cancer remained stable. Among women, a remarkable shift took place in the period under study. Cervical cancer was the most common cancer in 1995-1998, but it was reduced by half in 2008-2012. The incidence of breast cancer increased by 76%, and this was the most common cancer in women in 2008-2012. The incidence of stomach and liver cancer decreased in both sexes.
FIG 1.
(A) Incidence rates (per 100,000) of specific cancers in men in 2008-2012 and 1995-1998 in Ho Chi Minh City. (B) Incidence rates (per 100,000) of specific cancers in women in 2008-2012 and 1995-1998 in Ho Chi Minh City.
The overall incidence of cancer in Hanoi during 1993-1997 was 151.7 per 100,000 in men and 99.1 per 100,000 in women (6% higher than in HCMC in the same period in men and 9% lower in women). The incidence of lung cancer in men was 28% higher in Hanoi than in HCMC, whereas the incidence of liver cancer was 26% lower. Among women, the incidence of breast cancer was 54% higher in Hanoi than in HCMC, that of cervical cancer was 42% lower, and that of lung cancer was 4% lower (data not shown).
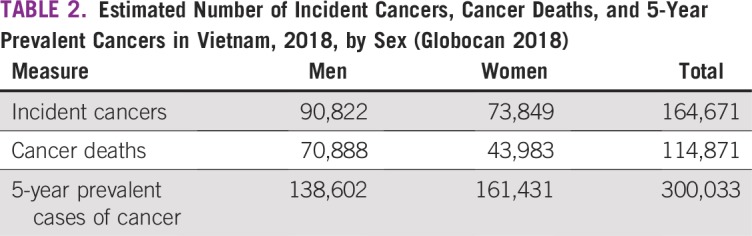
The estimated number of cancers in 2018 in Vietnam was 164,671 (55% in men). The estimated number of cancer deaths was 114,871 (62% in men), and the number of 5-year prevalent cases of cancer was 300,033 (Table 2). The top 5 causes of cancer death in 2018 were liver cancer (25,404 estimated deaths, 22.1% of the total), lung cancer (20,710 deaths, 18.0%), stomach cancer (15,065 deaths, 13.1%), breast cancer (6,103 deaths, 5.3%), and leukemia (4,923 deaths, 4.3%; data not shown).
TABLE 2.
Estimated Number of Incident Cancers, Cancer Deaths, and 5-Year Prevalent Cancers in Vietnam, 2018, by Sex (Globocan 2018)
Estimation of AFs
Estimation of AFs by risk factors in Vietnam is provided in Table 3. Infectious agents (29.1%) and tobacco smoking (13.5%) were accountable for the largest proportion of cancers in Vietnam. Among men, chronic infections accounted for one third of cancers, whereas tobacco smoking accounted for one fourth of all cancers. One of every 10 cancers could be attributed to drinking alcohol. In women, the 2 leading risk factors were infectious agents (22.1%) and passive smoking (8.8%). The remaining factors included in the analysis accounted for a relatively small fraction of cancers. Overall, the risk factors selected for the analysis were responsible for 59.2% of cancers in men and 32.0% in women (47% in both sexes).
TABLE 3.
Cancers Attributable to Selected Risk Factors in Vietnam in 2018
Table 4 lists the summary results of AFs for individual cancers. EBV, human papillomavirus, and HIV accounted for 100% of nasopharyngeal cancers, cervical cancers, and Kaposi sarcomas, respectively. Large AFs (≥ 70%) were observed for cancers strongly associated with tobacco smoking, alcohol drinking, and chronic infections (eg, head and neck, cervical cancers, liver, stomach). For lung cancer, the AF was 65%, with a large difference between men and women. AFs in the range of 20% to 60% were observed for cancer with ≥ 1 important, but not predominant, risk factor (eg, esophagus, lymphoma, reproductive system, bladder, and pancreas). AFs < 10% were observed for several common cancers (eg, colorectum, gallbladder, prostate, kidney, and ovary), for which the etiology is largely unknown.
TABLE 4.
Cancers Attributable to Known Risk Factors by Type of Cancer, Vietnam 2018

DISCUSSION
To our knowledge, this analysis was the first systematic assessment of cancers attributable to modifiable risk factors in Vietnam. Cancer incidence in Vietnam observed a minor decline over a 20-year period. Overall, cancer morbidity and mortality were higher among men than women. Lung cancer remained the most common cancer among men over the years, whereas breast cancer replaced cervical cancer to become the most common cancer among women. Cancers related to infectious agents (ie, stomach, liver, and cervical cancer) decreased, whereas cancers related to nutrition and metabolism (ie, breast, ovarian, and colorectal cancer) were on the rise. This changing pattern reflects the changes in risk factor prevalence for Vietnam, a country undergoing socioeconomical development, rapid urbanization, and lifestyle changes. This pattern is similar to China’s21 and that of most other Asian countries.22
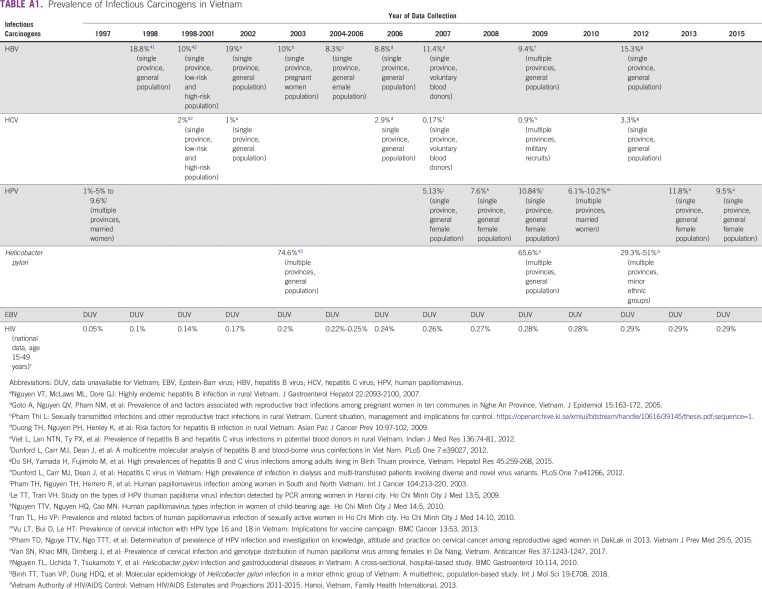
Infectious agents are the most common cancer risk factors in Vietnam. In our analysis, we could not include several relatively minor oncogenic infections, such as Opisthorchis riverine, Clonorchis sinensis, human herpesvirus 8, and liver flukes because of lack of exposure data. Chronic infections had a larger contribution to the total number of cancers in Vietnam than in global analyses (23.4% of total cancers in less developed region23), regional analyses (19.6% in Asian countries20), and results from other Asian countries such as China (25.3%),21 Japan (17.5%),24 and Korea (17.8%).20 This disparity could be explained by the fact that Vietnam is one of the countries with the highest prevalence of hepatitis B virus (10%)25 and H pylori (> 70%)26 in Asia. There is no national surveillance to monitor the trend of carcinogenic infections over time, but ad hoc epidemiologic studies showed a decreasing trend for most infectious agents included in our analysis (Appendix Table A1). These trends parallel those observed for infection-related cancers such as liver, stomach, and cervical cancer (Figs 1A and 1B).
Tobacco smoking is the second leading cause for cancer in Vietnam. Notably, > 97% of cancers caused by tobacco smoking occurred among men, reflecting a low tobacco smoking prevalence in women (1.9%) compared with men (60.1%).27 Despite the low prevalence of active smoking, passive smoking is a significant cancer risk factor for women in Vietnam, causing 8.8% of all cancers among women. Overall, smoking-related cancers accounted for one fifth of all cancers in Vietnam (27.5% in men and 9.6% in women). Our findings might underestimate the true burden of tobacco-related cancer in Vietnam because cancers with established but weak association with tobacco smoking (eg, colorectal and kidney cancer) were not included in the analysis. Ever-smoking as cancer risk factor accounted for a significantly smaller proportion of cancers among women in Vietnam (0.8%) than among women of other Asian populations (5% in China21 and 5.2% in Korea28 and Japan29). This could be explained by the low rate of active smoking among Vietnamese women (1.9%)27 compared with that of women in other studies (10.5% in Japan29 and 4.1% in Korea28). Smoking prevalence in Vietnam is decreasing slowly, by only 5% over a 10-year period27; hence, tobacco-related cancer will remain a major burden in the future.
Alcohol drinking was the third most important risk factor for cancer in Vietnam included in our analysis and accounted for 6% of cancers. Similar to tobacco smoking, a majority of alcohol drinking–related cancers (95.2%) occurred among men. Our estimation is higher than results from studies in China (3.63%30 to 4.4%21 of cancers caused by drinking alcohol) and previous global studies (3%-5% of cancer deaths caused by drinking alcohol across the globe).31,32 The risk of developing cancer increases with higher levels of drinking.32 Studies in China did not disaggregate by alcohol consumption level, which may lead to underestimation of AFs. However, differences in AFs for alcohol drinking could mainly be explained by a remarkable higher prevalence of alcohol consumption among men in Vietnam (82% in Vietnam compared with 39% in China30 and 55% globally32). Strikingly, alcohol consumption in Vietnam observed a remarkable increase of 21% between 2010 and 2015,33 suggesting that alcohol consumption may result in a more significant burden of cancer in the future.
Because of lack of exposure data, we excluded most of the occupational and environmental agents in our analysis; however, we could estimate the impact of outdoor air pollutants (ie, level of PM2.5) on cancer in Vietnam. Few studies in the past have evaluated such attribution. A similar study in France estimated that only 0.2% of all cancer deaths were caused by air pollutants.34 Further studies in this area in Vietnam are warranted.
Other lifestyle and reproductive factors included in this analysis contributed little to the cancer burden in Vietnam. Because of lack of data on hormonal, reproductive, and nutritional factors, we likely underestimated the impact of such risk factors in Vietnam. Our estimation for AFs of overweight and obesity (0.8%) was much lower than that of developed countries (ie, 1.6% in France,34 5.3% in very high HDI countries, and 4.8% in high HDI countries35). We used the prevalence of overweight and obesity in 2010 for the analysis, and a prompt growth in the prevalence was observed in recent years, with an increase from 12% in 2010 to 23.9% in 2015,33 indicating the possibility of a larger contribution of overweight and obesity to the burden of cancer in Vietnam in the future. Low fruit and vegetable intake played a small role in total cancer burden in Vietnam, accounting for just 0.4% of all cancers. Globally, consuming an insufficient amount of fruit and vegetables daily could cause 14% of GI cancer deaths worldwide.36 Other dietary factors potentially associated with cancer14 (eg, red meat intake, salting-preserved food consumption, high-dose β-carotene supplements) were not included in our study, which might hamper our current analysis on diet and nutrition component. Nulliparity is the only reproductive factor included in this analysis and accounted for < 2% of all cancers in women; again, underestimation of the role of reproductive and hormonal factors on the cancer burden in Vietnam is likely to have occurred.
Although we understand well the role of infections and tobacco smoking in cancer, leading to opportunities for cancer prevention in the future, we understand less well the causes of hormonal and metabolic-related cancers. Because these factors are emerging in societies under transition, such as Vietnam, it is important to conduct further studies to investigate their contribution to cancer in the country.
Our study has several strengths, including being the first effort, to our knowledge, to systematically assess the role of established cancer risk factors in Vietnam. In addition, most of the exposure data included in our analysis were derived from large-scale representative studies of the Vietnamese population. However, there were several drawbacks to our study. First, cancer diagnosis in Vietnam has many challenges. Access to quality pathology services has been limited in the past. Modern technologies for cancer diagnosis have become available in a few comprehensive cancer centers in recent years.37 In addition, the accuracy of pathology testing is also limited.38 These limitations may hinder the accuracy of data for the cancer registries. Second, we lack data for several important established risk factors. Third, some prevalence of exposure either might not be representative of the country (ie, infectious agents) or was based on the estimation outside of Vietnam (ie, exposure to outdoor pollution). Fourth, risk factors were referring to a time period that may not be fully relevant to cancer development. Finally, RRs were obtained from non-Vietnamese population studies, which may lead to potential under- or overestimation of certain risk factors specifically for the Vietnamese population.
Overall, approximately half of cancers in Vietnam can be attributed to known risk factors and therefore can be prevented, at least in theory. The effect of risk factors excluded from the analysis on the burden of cancer in Vietnam remains unknown. Therefore, it is important to monitor the exposures to such risk factors and to study the effect of these factors on cancer because there are no robust studies on the association between different risk factors and cancer in Vietnam currently available. Our findings once again confirmed the notion that controlling oncogenic infections and decreasing tobacco smoking are the most effective approaches to reduce the burden of environmental cancer in Vietnam, but other risk factors, including alcohol use, diet, and indoor and outdoor air pollution should not be neglected.
Appendix
TABLE A1.
Prevalence of Infectious Carcinogens in Vietnam
AUTHOR CONTRIBUTIONS
Conception and design: Thuy Phuong Nguyen, Hung N. Luu, Paolo Boffetta
Collection and assembly of data: All authors
Data analysis and interpretation: Thuy Phuong Nguyen, Hung N. Luu, Thuy Thi Van Tuong, Chi Thi Du Tran, Paolo Boffetta
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.The World Bank Vietnam at a glance. http://www.worldbank.org/en/country/vietnam/overview
- 2.WHO Vietnam country profile. https://www.who.int/countries/vnm/en/
- 3.Institute for Health Metrics and Evaluation . Findings from the Global Burden of Disease Study 2017. Seattle, WA: Institute for Health Metrics and Evaluation; 2018. [Google Scholar]
- 4.Van Hoang D, Lee AH, Pham NM, et al. Prostate cancer risk reduced by physical activity even among men with prolonged sitting time: A study from Vietnam. Asia Pac J Public Health. 2018;30:227–234. doi: 10.1177/1010539518756980. [DOI] [PubMed] [Google Scholar]
- 5.Binh TT, Tuan VP, Dung HDQ, et al. Advanced non-cardia gastric cancer and Helicobacter pylori infection in Vietnam. Gut Pathog. 2017;9:46. doi: 10.1186/s13099-017-0195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai HT, Koriyama C, Tokudome S, et al. Waterpipe tobacco smoking and gastric cancer risk among Vietnamese men. PLoS One. 2016;11:e0165587. doi: 10.1371/journal.pone.0165587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. doi: 10.22034/APJCP.2017.18.10.2747. Trieu PD, Mello-Thoms C, Peat JK, et al: Inconsistencies of breast cancer risk factors between the northern and southern regions of Vietnam. Asian Pac J Cancer Prev 18:2747-2754, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bray F, Colombet M, Mery L, et al: Cancer Incidence in Five Continents Volume XI. International Agency for Research on Cancer. http://ci5.iarc.fr.
- 9. Parkin DM, Whelan SL, Ferlay J, et al: Cancer Incidence in Five Continents, Volume VIII. Lyon, France, International Agency for Research on Cancer, 2002. [Google Scholar]
- 10. Ferlay J, Ervik M, Lam F, et al: Global Cancer Observatory (GCO): Cancer today. International Agency for Research on Cancer. https://gco.iarc.fr/today.
- 11.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 12.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 13. International Agency for Research on Cancer: Agents classified by the IARC monographs, volumes 1–123. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. https://monographs.iarc.fr/agents-classified-by-the-iarc/
- 14. World Cancer Research Fund, American Institute for Cancer Research: Continue Update Project: Diet, nutrition, physical activity and the prevention of cancer—Summary of strong evidence. https://www.wcrf.org/sites/default/files/Matrix-for-all-cancers-A3.pdf.
- 15.Opdahl S, Alsaker MDK, Janszky I, et al. Joint effects of nulliparity and other breast cancer risk factors. Br J Cancer. 2011;105:731–736. doi: 10.1038/bjc.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fioretti F, Tavani A, Bosetti C, et al. Risk factors for breast cancer in nulliparous women. Br J Cancer. 1999;79:1923–1928. doi: 10.1038/sj.bjc.6690306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–541. [PubMed] [Google Scholar]
- 18.Hanley JA. A heuristic approach to the formulas for population attributable fraction. J Epidemiol Community Health. 2001;55:508–514. doi: 10.1136/jech.55.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Hu X-F, Zhao F-H, et al. Estimation of cancer burden attributable to infection in Asia. J Epidemiol. 2015;25:626–638. doi: 10.2188/jea.JE20140215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang JB, Jiang Y, Liang H, et al. Attributable causes of cancer in China. Ann Oncol. 2012;23:2983–2989. doi: 10.1093/annonc/mds139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sankaranarayanan R, Ramadas K, Qiao YL. Managing the changing burden of cancer in Asia. BMC Med. 2014;12:3. doi: 10.1186/1741-7015-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob Health. 2016;4:e609–e616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 24.Inoue M, Sawada N, Matsuda T, et al. Attributable causes of cancer in Japan in 2005: Systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol. 2012;23:1362–1369. doi: 10.1093/annonc/mdr437. [DOI] [PubMed] [Google Scholar]
- 25.Razavi-Shearer D, Gamkrelidze I, Nguyen MH, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 26.Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 27. Ministry of Health of Vietnam, Hanoi Medical University, General Statistics Office, et al: Global Adult Tobacco Survey (GATS) Vietnam 2010. https://www.who.int/tobacco/surveillance/en_tfi_gats_vietnam_report.pdf.
- 28.Park S, Jee SH, Shin H-R, et al. Attributable fraction of tobacco smoking on cancer using population-based nationwide cancer incidence and mortality data in Korea. BMC Cancer. 2014;14:406. doi: 10.1186/1471-2407-14-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katanoda K, Marugame T, Saika K, et al. Population attributable fraction of mortality associated with tobacco smoking in Japan: A pooled analysis of three large-scale cohort studies. J Epidemiol. 2008;18:251–264. doi: 10.2188/jea.JE2007429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang H, Wang J, Xiao H, et al. Estimation of cancer incidence and mortality attributable to alcohol drinking in China. BMC Public Health. 2010;10:730. doi: 10.1186/1471-2458-10-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danaei G, Vander Hoorn S, Lopez AD, et al. Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 32.Boffetta P, Hashibe M, La Vecchia C, et al. The burden of cancer attributable to alcohol drinking. Int J Cancer. 2006;119:884–887. doi: 10.1002/ijc.21903. [DOI] [PubMed] [Google Scholar]
- 33. Ministry of Health of Vietnam: National Survey on the Risk Factors of Non-Communicable Diseases (STEPS) Viet Nam 2015. https://www.who.int/ncds/surveillance/steps/VietNam_2015_STEPS_Report.pdf.
- 34.Boffetta P, Tubiana M, Hill C, et al. The causes of cancer in France. Ann Oncol. 2009;20:550–555. doi: 10.1093/annonc/mdn597. [DOI] [PubMed] [Google Scholar]
- 35.Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO Promoting fruit and vegetable consumption around the world. https://www.who.int/dietphysicalactivity/fruit/en/index2.html
- 37.Nguyen BD. Results of the national cancer program 2011-2014. Vietnam Oncol J. 2014;2:8. [Google Scholar]
- 38.Pham T, Bui L, Kim G, et al. Cancers in Vietnam: Burden and control efforts—A narrative scoping review Cancer Control 2610732748198638022019. CHECK PAGE REF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO Fact sheet about health benefits of smoking cessation. Tobacco Free Initiative (TFI) https://www.who.int/tobacco/quitting/benefits/en/
- 40.Van Bui T, Blizzard CL, Luong KN, et al. Alcohol consumption in Vietnam, and the use of “standard drinks” to measure alcohol intake. Alcohol Alcohol. 2016;51:186–195. doi: 10.1093/alcalc/agv082. [DOI] [PubMed] [Google Scholar]
- 41.Hipgrave DB, Nguyen TV, Vu MH, et al. Hepatitis B infection in rural Vietnam and the implications for a national program of infant immunization. Am J Trop Med Hyg. 2003;69:288–294. [PubMed] [Google Scholar]
- 42.Tran HT-T, Ushijima H, Quang VX, et al. Prevalence of hepatitis virus types B through E and genotypic distribution of HBV and HCV in Ho Chi Minh City, Vietnam. Hepatol Res. 2003;26:275–280. doi: 10.1016/s1386-6346(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 43.Hoang TTH, Bengtsson C, Phung DC, et al. Seroprevalence of Helicobacter pylori infection in urban and rural Vietnam. Clin Diagn Lab Immunol. 2005;12:81–85. doi: 10.1128/CDLI.12.1.81-85.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vietnam Administration of HIV/AIDS Control: Vietnam HIV/AIDS estimates and projections 2007-2012. http://www.ilo.org/wcmsp5/groups/public/---ed_protect/---protrav/---ilo_aids/documents/legaldocument/wcms_174609.pdf.
- 45.Brauer M, Freedman G, Frostad J, et al. Ambient air pollution exposure estimation for the global burden of disease 2013. Environ Sci Technol. 2016;50:79–88. doi: 10.1021/acs.est.5b03709. [DOI] [PubMed] [Google Scholar]
- 46. Committee for Population Family and Children: Vietnam Demographic and Health Survey 2002. https://dhsprogram.com/pubs/pdf/fr139/00frontmatter00.pdf.