Abstract
BACKGROUND AND PURPOSE: Meningeal inflammatory myofibroblastic tumor (IMT) has been rarely reported, and its prognosis is still unclear. Our purpose was to describe the imaging features of patients with meningeal IMT and their results on follow-up studies.
MATERIALS AND METHODS: Twenty-four MR images in 10 consecutive patients with pathologically proved meningeal IMTs were retrospectively evaluated, focusing on the lesion distribution, signal intensity (SI), and contrast-enhancement pattern with a review of the clinical records.
RESULTS: Eight patients with intracranial IMT showed localized (n = 4) or diffuse (n = 4) dural thickening, a single mass (n = 5) or 2 (n = 2) dural-based masses with surrounding edema, dural venous sinus thrombosis (n = 5), and leptomeningeal involvement (n = 5). Extracranial involvement of the mastoid (n = 2) and orbit (n = 2) was also associated. Each of the 2 patients with intraspinal IMT showed a dural-based mass and a segmental dural thickening, respectively. All of the thickened dura showed low SI on T2-weighted images, iso-SI on T1-weighted images, and diffuse contrast enhancement. Variable recurrences with dural-based masses, mastoid involvement, or nasolacrimal duct involvement were observed in all 4 patients with diffuse intracranial IMT, but not in the others.
CONCLUSIONS: Localized or diffuse dural thickening of T2 low SI and diffuse contrast enhancement combined with dural-based masses are a common MR imaging finding of meningeal intracranial IMT. Adjacent leptomeningeal involvement and dural venous sinus thrombosis are frequently associated. The diffuse type has a tendency toward recurrence.
An inflammatory myofibroblastic tumor (IMT) is an enigmatic neoplasm of mesenchymal origin, and its pathologic definition had been disputed for many years until it was finally allocated to the soft-tissue tumor category in the 2002 WHO classification of soft-tissue tumors.1–4 Histopathologically, it is characterized by the proliferation of myofibroblastic cells with variable proportions of inflammatory infiltrates, collagenous stroma, and fibrosis. The inflammatory cells are mature and polyclonal and are mixed with plasma cells, lymphocytes, eosinophils, and histiocytes.2,4,5 IMTs have been described with many different names because of the varying histologic proportions and clinical features.2,5
Although IMT most commonly involves the lung and the orbit, it can occur in any site in the body and sometimes, albeit rarely, involves intracranial and intraspinal structures. However, when it does involve these sites, it has been reported to occur most commonly in the meninges out of various sites, including the brain parenchyma, ventricle, hypothalamus, sellar region, choroid plexus, skull base, and spine.6–9 Meningeal IMT has been rarely reported using the terms “inflammatory pseudotumor” and “plasma cell granuloma,” and the prognosis is still unclear.6,7,10
The purpose of the present study was to describe the imaging features of patients with meningeal IMT and the results on follow-up studies.
Materials and Methods
Patients
The institutional review board of our hospital approved this study and waived informed consent. Our retrospective study included 10 consecutive patients with meningeal IMTs (8 patients with intracranial IMT and 2 patients with intraspinal IMT) from a computerized search of the pathologic, radiologic, and medical records of our institution from 1996 to 2006. At the time of initial meningeal manifestation, the patients’ ages ranged from 17 to 67 years (7 males and 3 females; mean age, 44 years). For a consecutive study focusing on the radiologic details of the meningeal IMTs, 5 (patients 3–6 and 9 in the on-line Table) of 10 patients who had been enrolled in a prior study of Jeon et al4 were included in our study after revision of radiologic-clinical analysis.
Histologic Examination
All pathologic specimens were obtained through either surgical resection (n = 8) or biopsy (n = 2), resection of a dural-based mass (n = 4) or a dural thickening (n = 1), resection of a dural-based mass with mastoidectomy (n = 2) and combined with thrombectomy (n = 1), a biopsy of the thickened dura (n = 1), a biopsy of a dural-based mass and 2 subsequent biopsies of recurrent medial canthal masses (n = 1).
One pathologist (S.-H.P.) reviewed the hematoxylin-eosin (HE)–stained slides in each case to reconfirm the morphologic diagnosis of IMTs by using the criteria of spindle-shaped myofibroblastic mesenchymal cells with variable degrees of fibrosis and inflammatory cells. Upon review of the immunohistochemistry with a focus on the expression of smooth muscle actin (SMA) and anaplastic lymphoma kinase (ALK), all the cases showed positive expression of SMA, but negative expression of ALK. There was no significant pathologic difference between localized intracranial IMTs and diffuse intracranial IMTs.
Clinical Data
Clinical records were available in all cases and were reviewed by 1 neuroradiologist (J.-H.K.) with regard to age, sex, clinical presentation with past medical history, and disease course including the response to therapy. The following data were obtained from laboratory tests at initial meningeal manifestation or recurrence during follow-up, when available: CSF protein level, glucose concentration, and white blood cell (WBC) count. Clinical follow-up duration ranged from 3 months to 11 years (mean, 3.9 years).
Imaging
Twenty-four MR examinations were performed at field strengths of 0.5T (Gyrex; Elscint, Haifa, Israel), 1T (Magnetom Expert; Siemens, Erlangen, Germany), 1.5 T (Signa, GE Healthcare, Milwaukee, Wis; or Magnetom Vision Plus, Siemens) or 3T (Signa, GE Healthcare) in 10 patients (1–5 images per patient; mean, 2.4 images). MR imaging sequences included spin-echo T1-weighted images (T1WI; TR/TE/NEX, 400–644/8–20/2–3), fast spin-echo T2-weighted images (T2WI; TR/TEeff/NEX, 3200–5000/85–129/1–3), and contrast-enhanced T1WI in multiple planes. Contrast-enhanced T1WIs were obtained after intravenous administration of 0.1-mmol/kg gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany) in all MR images. MR venography (both 2D time-of-flight and 3D phase-contrast) and conventional digital subtraction angiography were performed in 4 patients. Twelve CT examinations were performed in 4 patients; 4 precontrast brain CTs, in 4 patients; 3 pre- and postcontrast brain CTs, in 3 patients; 3 high-resolution temporal bone CTs (s), in 3 patients; and 2 postcontrast CTs for the orbit and paranasal sinus, in 1 patient. The time interval between the first and the last radiologic images ranged from 0 to 11 years (mean, 3.2 years).
Image Analysis
Two neuroradiologists (J.-H.K., K.-H.C.) retrospectively reviewed all of the radiologic images with respect to dural thickening, dural-based mass, dural venous sinus thrombosis, leptomeningeal involvement, and extracranial involvement, focusing on distribution, MR signal intensity (SI), CT attenuation, and contrast-enhancement pattern at MR imaging and CT.
Intracranial IMT was classified into localized and diffuse types according to the extent of intracranial dural thickening. When the dural thickening involved 1 or 2 adjacent convexities of 4 bilateral cerebral and cerebellar convexities, it was defined as “localized type.” If the dural thickening involved 3 or 4 convexities, it was defined as “diffuse type.” The presence of dural venous sinus thrombosis was evaluated at the superior sagittal sinus, transverse sinus, and sigmoid sinus. Leptomeningeal involvement was considered positive when abnormal enhancement in the cortical sulci adjacent to the dural-based mass or dural thickening, appearing as a spiculated or serrated margin of the thickened dura, was demonstrated. On MR imaging and CT, lesion SI and attenuation were compared qualitatively with those of normal cerebral gray matter and were determined at the relatively most dominant portion. The interval changes of the lesions on follow-up images were also evaluated.
Results
Clinical and radiologic features of 10 patients with meningeal IMTs are summarized in the Table. Ten patients with meningeal IMT comprised 8 patients with intracranial IMT (localized dural thickening, patients 1–4; diffuse dural thickening, patients 5–8) and 2 patients with intraspinal IMT (patients 9 and 10).
Clinical and Laboratory Features
In patients with intracranial IMT, headache and seizures were the most common symptoms (4/8; 50%, respectively) followed by blindness (2/8, 25%). In patients with localized intracranial IMT, seizure was the most common symptom (3/4; 75%). Other various neurologic symptoms were dependent on the involved sites.
CSF protein was elevated in 4 (80%) of 5 patients examined, but CSF glucose concentration was within the normal range in all 5 patients (100%). There was a mild increase in CSF WBC count (range, 6–40/mm3) with lymphocyte dominancy in 3 patients (60%) and no WBC in 2 patients (40%) of 5 patients. Serologic studies suggesting infection revealed no abnormalities in all patients.
Radiologic Features
Initial Imaging Features.
In intracranial IMT (n = 8), besides localized (n = 4) or diffuse (n = 4) dural thickening, 9 dural-based masses with surrounding edema were initially found in 7 patients: 1 mass in 5 patients each and 2 in 2 patients each (Figs 1 and 2). Other intracranial findings included dural venous sinus thrombosis (n = 5) and leptomeningeal involvement (n = 5) (Figs 1 and 2). Extracranial involvement was also associated in the mastoid (n = 2) and orbit (n = 2) (Figs 1 and 2). The 2 patients with intraspinal IMT showed a dural-based mass and a segmental dural thickening, respectively (Fig 3).
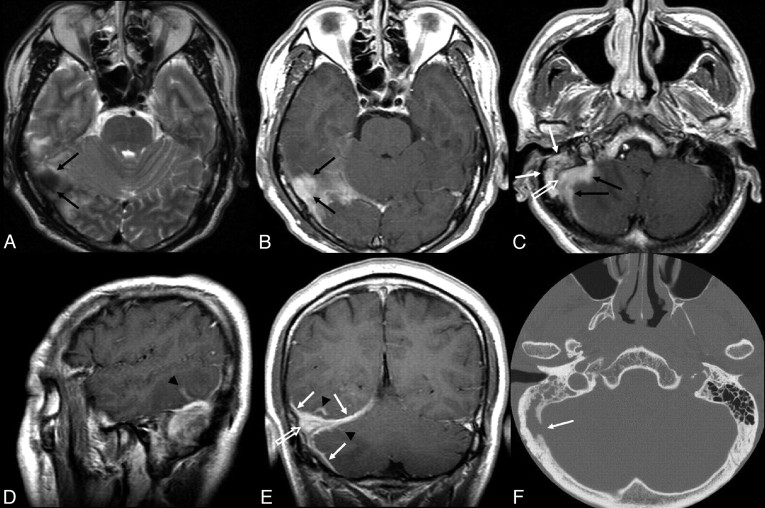
Fig 1.
Patient 4. A localized intracranial meningeal inflammatory myofibroblastic tumor in a 41-year-old man. A, Transverse T2-weighted MR image shows a nodular dural-based mass (black arrows) with low SI at the right occipital area. B, Transverse postcontrast T1-weighted MR image shows homogeneous enhancement of the mass (black arrows). C, Transverse postcontrast T1-weighted MR image shows homogeneous enhancement of another spiculated dural-based mass (black arrows) at the right cerebellar convexity and the adjacent sigmoid sinus (white open arrow), with heterogeneous enhancement of the right mastoid (white arrows). D and E, Sagittal (D) and coronal (E) postcontrast T1-weighted MR images show homogeneous enhancement of the right transverse sinus (white open arrow) and the enhancing thickened dura (white arrows) and leptomeningeal enhancement (arrowheads) at the adjacent tentorium, occiptal, and cerebellar convexities. F, Transverse temporal bone CT shows the destruction of the right sigmoid plate (white arrow).
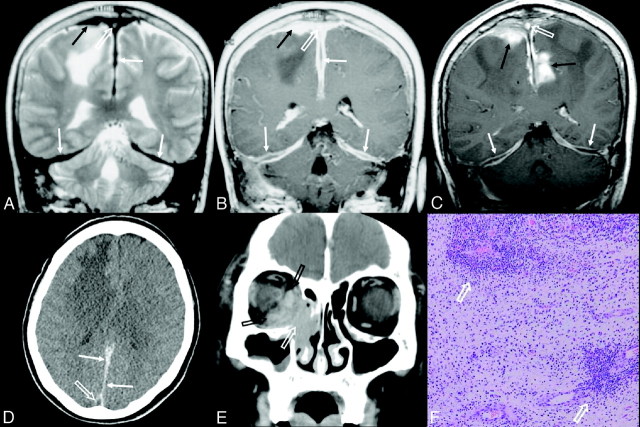
Fig 2.
Patient 6. A diffuse intracranial meningeal inflammatory myofibroblastic tumor in a 60-year-old woman showing multiple recurrences. A and B, At initial meningeal manifestation, coronal T2- (A) and postcontrast T1-weighted (B) images show diffuse dural thickening (white arrows) of T2 low SI with a nonenhancing central linear area in the enhancing thickened falx cerebri and bilateral tentorium, a homogeneously enhancing en plaque dural-based mass (black arrows) with extensive edema at the right frontal lobe, and dural venous sinus thrombosis with heterogenous T2 high SI and homogeneous enhancement at the superior sagittal sinus (white open arrows). C, Two years later, coronal postcontrast T1-weighted image shows an increase of the dural-based mass at the right frontal parasagittal convexity and a new development of the enhancing spiculated mass at the left parietal parasagittal area (black arrows), with persistent dural venous sinus thrombosis at the superior sagittal sinus (white open arrow). The enhancing thickened falx and tentorium show a decreased enhancement of the peripheral margin and an increased nonenhancing central linear area (white arrows). D, Transverse precontrast CT image shows diffuse thickening of posterior falx cerebri (white arrows) with high attenuation. The superior sagittal sinus (white open arrow) shows low attenuation and appears as a pseudoempty delta from the surrounding dural thickening. E, Four years after the initial meningeal manifestation, coronal orbit CT scan shows a homogeneously enhancing mass at the right nasolacrimal duct and adjacent right inferomedial orbital area (white open arrow) with destruction of the lamina papyracea. The inferior and medial rectus muscles are enlarged (black open arrows). F, Photomicrograph obtained at the right frontal dural-based mass from B shows multifocal areas of the predominant lymphoplasma cell infiltrations (white open arrows) in fibrovascular stroma mixed with myofibroblastic proliferation and a minor population of mixed inflammatory cells (HE, original magnification ×100).
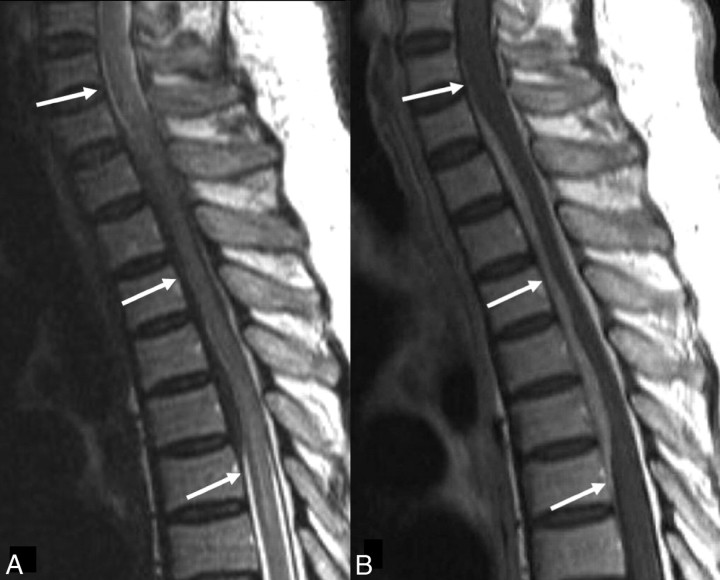
Fig 3.
Patient 10. A spinal inflammatory myofibroblastic tumor in a 63-year-old man. A, Sagittal T2-weighted image shows a segmental dural thickening at the C7-T6 level (white arrows). B, Sagittal T1-weighted image shows a segmental dural thickening with homogeneous enhancement at the C7-T6 level (white arrows).
Intracranial IMT
Dural Thickening and Dural-Based Mass Lesions.
In 4 patients with localized IMT, dural thickening was found in the unilateral cerebral convexity (2/4, 50%), the unilateral cerebral convexity with adjacent falx cerebri (1/4, 25%), and the unilateral tentorium cerebelli combined with adjacent cerebral and cerebellar convexities (1/4, 25%) (Fig 1). In all 4 patients with diffuse IMT, the dural thickening involved bilateral cerebral and cerebellar convexities along with the falx cerebri and the bilateral tentorium cerebelli (4/4, 100%), and it showed a nonenhancing central linear area within the enhancing thickened falx and tentorium (4/4, 100%) (Fig 2).
All 13 dural-based masses, which were found initially (n = 9) or during follow-up (n = 4) in 8 patients with intracranial IMT, involved cerebral (10/13; 77%) or cerebellar convexities (3/13, 23%) (Figs 1 and 2). At MR imaging, all the 8 thickened duras and the 13 dural-based masses showed the following features: low SI on T2WI (8/8, 100%; 9/13, 69%), iso-SI on T1WI (8/8,100%; 12/13, 92%), and diffuse enhancement (8/8, 100%; 13/13,100%), respectively (Figs 1 and 2). At CT, most of the thickened duras and dural-based masses showed high attenuation (4/4, 100%; 6/7, 86%) and diffuse enhancement (3/3, 100%; 2/2, 100%) (Fig 2).
During steroid treatment in 4 patients with diffuse IMT, the thickening and contrast enhancement of the dura decreased in degree with little change in their overall extent (4/4, 100%), but the nonenhancing central linear area within the enhancing thickened dura was not changed (2/4, 50%) or rather increased (2/4, 50%) in extent (Fig 2). During steroid treatment in 4 patients with diffuse IMT, the 5 dural-based mass lesions (5/8, 63%) in 2 patients (patients 5 and 6) decreased in size. However, 1 lesion (1/8, 13%) re-increased in size (patient 7), and 2 lesions (2/8, 25%) newly occurred (patient 8). Their sizes decreased with repeated steroid treatment (3/3, 100%). The other 5 dural-based masses in the 4 patients with localized IMT disappeared after resection and steroid treatment.
Thrombosis of the Dural Venous Sinus.
Dural venous sinus thrombosis was initially seen in 1 patient with localized IMT (1/4, 25%) and in all 4 patients with diffuse IMT (4/4, 100%). It involved the right sigmoid and transverse sinuses (n = 5), the superior sagittal sinus (n = 4), and the left sigmoid and transverse sinuses (n = 1) (Figs 1 and 2). At MR imaging, they showed high (4/5, 80%) SI on T2WI, iso-SI (5/5, 100%) on T1WI, and homogeneous contrast enhancement (5/5, 100%) (Figs 1 and 2). At CT, they showed low attenuation and appeared as pseudoempty deltas in contrast to the adjacent high-attenuating dura (4/4, 100%) with heterogeneous (2/3, 67%) contrast enhancement (Fig 2). The thromboses at MR imaging were confirmed by MR venography and digital substraction angiography performed in all 4 patients. The extent of dural venous sinus thrombosis did not change during anticoagulation in all 4 patients with diffuse IMT (Fig 2). In the 1 patient with localized IMT, the venous sinus thrombosis was surgically treated (Fig 1).
Leptomeningeal Involvement.
Leptomeningeal involvement was initially seen at MR imaging in 5 patients (3 of 4 patients with localized IMT and 2 of 4 patients with diffuse IMT; 3 of 5 patients with dural venous sinus thrombosis and 2 of 3 patients without dural venous sinus thrombosis) (Fig 1). All the leptomeningeal involvement, however, disappeared after surgery and steroid treatment in 3 patients with localized IMT and after steroid treatment alone in 2 patients with diffuse IMT.
Localized Brain Parenchymal Edema.
All 13 dural-based masses were associated with surrounding edema (13/13, 100%) (Figs 1 and 2). The parenchymal edemas of 10 dural-based masses in 5 patients coexisted with dural venous sinus thrombosis, but those of 3 dural-based masses in 3 patients did not.
Extracranial Involvement.
Extracranial involvement was seen in 1 of 4 patients with localized IMT (1/4, 25%), whereas it was noted in all 4 patients with diffuse IMT (4/4, 100%).
The mastoid was involved in 3 patients (1 with localized IMT [1/4, 75%] and 2 with diffuse IMT [2/4, 50%]) in whom dural venous sinus thrombosis was associated, 2 patients concurrently and 1 patient 5 years after initial meningeal manifestation and dural venous sinus thrombosis. There was soft-tissue filling at the right mastoid bone (3/3, 100%) with destruction of the mastoid air cells (3/3, 100%) and adjacent sigmoid plate (2/3, 67%) at temporal bone CT (TBCT) (Fig 1). At MR imaging, the mastoid lesions showed high SI on T2WI, iso-SI on T1WI, and heterogeneous enhancement (2/2, 100%) (Fig 1).
Orbital lesions were found in 2 patients with diffuse IMT (patients 5 and 7), in 1 of whom unilateral visual acuity was progressively lost 7 years before initial meningeal manifestation. The orbital lesions were closely approximate to the thickened dura and showed low SI on T2WI, low SI on T1WI, and poor contrast enhancement (2/2, 100%) at MR imaging. On CT, the orbital lesion showed high attenuation and heterogeneous contrast enhancement (1/1). The 2 orbital lesions did not significantly change in both extent and MR imaging SI or CT attenuation during their respective 11- and 3-year follow-ups.
In 1 patient with diffuse IMT (patient 6), 2 recurrent lesions involving the nasolacrimal duct and adjacent inferomedial orbit were associated with the destruction of adjacent lamina papyracea, which developed 4 and 6 years, respectively, after improvement of initially found dural-based masses (Fig 2).
Intraspinal IMT
Patients 9 and 10 showed an intradural extramedullary mass at the L1–2 vertebrae level and a segmental epidural mass from the C7 to T6 level, respectively. They showed low SI on T2WI, iso-SI on T1WI, and homogeneous MR imaging enhancement (2/2, 100%) (Fig 3).
Results on Follow-Up
All 4 patients with localized intracranial IMT were successfully treated with surgical resection and steroids without any recurrence during 2–5.5 years of follow-up (mean, 3.1 years). The 4 patients with diffuse intracranial IMT had the following recurrences during the 3–11 years of follow-up (mean, 6.3 years): 4 new dural-based masses in 3 patients (1 in 2 patients each and 2 in 1 patient), increased size of a pre-existing dural-based mass in 2 patients, 1 case of new mastoid involvement in 1 patient, and 2 recurrent lesions of the nasolacrimal duct and adjacent inferomedial orbit in 1 patient (Fig 2). They underwent multimodal therapies including steroid (n = 4), anticoagulation (n = 3), surgical resection (n = 3), radiation therapy (n = 2), and chemotherapy (n = 1). The 2 patients with intraspinal IMT who were treated with only surgical resection showed no evidence of recurrence on 3-month and 1-year follow-up.
Discussion
The meningeal preference of IMT in various intracranial and intraspinal structures may be related to the fact that the dura mater is an inert fibrous membrane with rich vascular networks lacking a blood-brain barrier in its outer and inner layers, closely related to the highly vascularized arachnoid.11
The most common symptoms in our patients with intracranial meningeal IMT were headache and seizure (50%, respectively), followed by blindness (25%). These results are similar to those reported by Greiner et al,7 for which headache was the most common symptom (52.6%), followed by visual disturbance (36.8%) and seizures (26.3%).
Regarding laboratory results, mild pleocytosis with lymphocyte dominancy, elevated protein, and normal glucose levels on CSF examinations in our study are concordant with the results reported in the literature and mimic aseptic meningitis.10
Like the dural lesions in the present study, IMT has been reported to show commonly low SI on both T2- and T1-weighted images.5,8,9 Low SI has been explained by the relative lack of free water and mobile protons and/or the presence of free radicals produced by macrophages during active phagocytosis within the lesions of rich fibrosis and collagenous stroma.8
Dural venous sinus thrombosis in all patients with diffuse IMT may be related to the anatomic location of the dural venous sinus between the inner and outer layers of the dura mater, which might be easily influenced by the chronic inflammatory processes of the dura. Frequently in patients with sigmoid sinus thrombosis, adjacent mastoids were concurrently or subsequently involved without clinical evidence of mastoiditis. This suggests that the mastoid lesion is not a cause but a consequence of dural venous sinus thrombosis in meningeal IMT, as Fink and McAuley12 argued in their study.
Leptomeningeal involvement does not appear to be related to dural venous sinus thrombosis or the extent of dural thickening. It was difficult to determine whether the etiology of localized brain parenchymal edema was from the direct effect of a dural-based lesion or dural venous sinus thrombosis. Although most of the dural-based masses showed parenchymal edema concurrently with dural venous sinus thrombosis, there were a few dural-based masses showing parenchymal edema without dural venous sinus thrombosis. Meanwhile, IMTs occurring at various extracranial locations in the head and neck have also been reported to extend intracranially to involve the meninges.5,8,13–15 The findings of chronic orbital lesions in 2 patients of the present study are consistent with prior reports on orbital IMT.5,13,14 Intracranial spread to the meninges of orbital pseudotumor was reported in 8 (8.9%) of 90 consecutive biopsy-proved cases.13
Like the 2 cases in the present study, intraspinal IMT has been reported as a mass or a segmental thickening of the dura, with varying locations: epidural, extramedullary intradural, and even intramedullary without dural attachment.9
Although the radiologic findings of dural thickening with or without dural-based mass, T2 low SI of enhancing dural lesions, and adjacent focal edema appear to be common in meningeal IMT, those features can overlap several other conditions, including fibroblastic en plaque meningioma, lymphoma, and idiopathic hypertrophic pachymeningitis.16–18 It is not easy to diagnose meningeal IMT on imaging features alone, but it should be considered in the differential diagnosis of focal and generalized dural thickening.
Histologic diagnosis of meningeal IMT may also be difficult. However, the immunohistochemical polyclonality of plasma cells may exclude the diagnosis of plasmacytoma and lymphoma.4,19 In addition, unlike the lymphoplasma cell-rich variant of meningioma, there is neither meningothelial proliferation nor epithelial membrane antigen expression in meningeal IMT.4,19 In our experience, idiopathic hypertrophic pachymeningitis may also be different from meningeal IMT in the fact that it usually shows unique abortive granulomas and contains multinucleated but small giant cells. Immunohistochemically, the stromal component of idiopathic hypertrophic pachymeningitis may not be myofibroblasts because most stromal cells are not immunoreactive for SMA.4
Although ALK overexpression was reported to occur in 35%–60% of overall IMT cases and in a few cases of recurred intracranial and spinal IMTs,6,20 it was not found in any of our 10 patients, not even in the 4 patients with recurrences in the present study. It still seems to be unclear whether IMT is a neoplasm or pseudotumor.
Although idiopathic hypertrophic pachymeningitis has been usually known to cause a dural thickening without a dural-based mass, several articles regarding idiopathic hypertrophic pachymeningitis reported radiologic findings similar to meningeal IMTs in the present study, in addition to the tumorlike behaviors.15,18,21–24 Particularly, the nonenhancing central linear area in the enhancing thickened dura of diffuse IMT in the present study had already been reported as a feature of idiopathic hypertrophic pachymeningitis, representing thickened dura with extensive fibrosis and peripheral inflammatory cell infiltration.21,25
In addition to similar clinical symptoms and laboratory findings, these similar radiologic features support the argument that both meningeal IMT and idiopathic hypertrophic pachymeningitis are part of a spectrum rather than discrete disorders, even if there were some pathologic differences between the 2 disease entities.10,21,23,25
As for treatment, surgical resection with adjuvant steroid medication is the treatment of choice for meningeal IMT.26,27 In our study, there was no recurrence in any patient with localized meningeal IMT following surgical resection with steroid treatment, but all the patients with unresectable diffuse IMT showed various types of recurrence despite the use of multimodal treatments.
In the present study, there was an overall 40% recurrence rate of meningeal IMT, which is concordant with prior results in which the recurrence rate of IMTs in the central nervous system was as high as 40% within 2 years in contrast to approximately a 15% recurrence rate reported in studies of extrapulmonary IMT.2,6 Central nervous system IMTs have been known to occur with various synchronous or metachronous lesions in other organs.6,7 However, recurrence at the nasolacrimal duct in patients with meningeal IMT, as seen in patient 6 in our study, has not, to our knowledge, been reported before.
Considering the 100% recurrence rate of diffuse meningeal IMT in this present study, one should closely monitor various intracranial or extracranial recurrences in patients with diffuse dural thickening, whether it is meningeal IMT or idiopathic hypertrophic pachymeningitis. It is our opinion that the recurrent tendency in the diffuse type may be related to the poor response to medical treatment and difficulty of the surgical treatment of diffuse dural thickening, compared with surgical excision in the localized type. In the present study, dural thickening and dural-based masses of patients with diffuse IMT were partially responsive to steroid treatment. There have been no well-established therapeutic strategy to prevent recurrence in such inoperable diffuse meningeal IMTs and no consensus on the proper dosage or duration of steroid treatment.26
The benefits of radiation and chemotherapy, which have been suggested as alternatives for cases of recurrence after optimal resection or regrowth after incomplete surgery and for residual tumors not responding to steroid therapy, have been limited.5,7,26,27 Regarding the dosage and duration of anticoagulation therapy, uncomplicated treatment of venous sinus thrombosis associated with meningeal IMT may also be another issue of concern.
In our study, we included only a small sample size due to the rarity of the tumor and a radiologic-clinical follow-up of limited duration. A larger meta-analysis or prospective study may be required.
In conclusion, localized or diffuse dural thickening with T2 low SI, T1 iso-SI, and diffuse contrast enhancement combined with single or multiple enhancing dural-based masses surrounded by brain parenchymal edema is a common MR imaging finding of meningeal intracranial IMT. Adjacent leptomeningeal involvement and dural venous sinus thrombosis are frequently associated. In addition, the diffuse type of meningeal intracranial IMT has a tendency to recur.
Footnotes
indicates article with supplemental on-line table.
References
- 1.Fletcher CDM, Unni K, Mertens F. Pathology and Genetics, Tumors of Soft Tissue and Bone: World Health Organization Classification of Tumors. Lyon, France: IARC Press;2002. :91–93
- 2.Coffin CM, Watterson J, Priest JR, et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor): a clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995;19:859–72 [DOI] [PubMed] [Google Scholar]
- 3.Polk P, Biggs PJ. Inflammatory tumor. Am J Surg Pathol 1996;20:899–900, author reply 901–02 [DOI] [PubMed] [Google Scholar]
- 4.Jeon YK, Chang KH, Suh YL, et al. Inflammatory myofibroblastic tumor of the central nervous system: clinicopathologic analysis of 10 cases. J Neuropathol Exp Neurol 2005;64:254–59 [DOI] [PubMed] [Google Scholar]
- 5.Narla LD, Newman B, Spottswood SS, et al. Inflammatory pseudotumor. Radiographics 2003;23:719–29 [DOI] [PubMed] [Google Scholar]
- 6.Hausler M, Schaade L, Ramaekers VT, et al. Inflammatory pseudotumors of the central nervous system: report of 3 cases and a literature review. Hum Pathol 2003;34:253–62 [DOI] [PubMed] [Google Scholar]
- 7.Greiner C, Rickert CH, Mollmann FT, et al. Plasma cell granuloma involving the brain and the lung. Acta Neurochir (Wien) 2003;145:1127–31. Epub 2003 Sep 22 [DOI] [PubMed] [Google Scholar]
- 8.Han MH, Chi JG, Kim MS, et al. Fibrosing inflammatory pseudotumors involving the skull base: MR and CT manifestations with histopathologic comparison. AJNR Am J Neuroradiol 1996;17:515–21 [PMC free article] [PubMed] [Google Scholar]
- 9.Seol HJ, Kim SS, Kim JE, et al. Inflammatory pseudotumor in the epidural space of the thoracic spine: a case report and literature review of MR imaging findings. AJNR Am J Neuroradiol 2005;26:2667–70 [PMC free article] [PubMed] [Google Scholar]
- 10.Tresser N, Rolf C, Cohen M. Plasma cell granulomas of the brain: pediatric case presentation and review of the literature. Childs Nerv Syst 1996;12:52–57 [DOI] [PubMed] [Google Scholar]
- 11.Meltzer CC, Fukui MB, Kanal E, et al. MR imaging of the meninges. Part I. Normal anatomic features and nonneoplastic disease. Radiology 1996;201:297–308 [DOI] [PubMed] [Google Scholar]
- 12.Fink JN, McAuley DL. Mastoid air sinus abnormalities associated with lateral venous sinus thrombosis: cause or consequence? Stroke 2002;33:290–92 [DOI] [PubMed] [Google Scholar]
- 13.Clifton AG, Borgstein RL, Moseley IF, et al. Intracranial extension of orbital pseudotumour. Clin Radiol 1992;45:23–26 [DOI] [PubMed] [Google Scholar]
- 14.de Jesus O, Inserni JA, Gonzalez A, et al. Idiopathic orbital inflammation with intracranial extension: case report. J Neurosurg 1996;85:510–13 [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto K, Natori Y, Hirokawa E, et al. Hypertrophic pachymeningitis as a result of a retropharyngeal inflammatory pseudotumor: case report. Neurosurgery 2002;51:1061–64, discussion 1064–65 [DOI] [PubMed] [Google Scholar]
- 16.Elster AD, Challa VR, Gilbert TH, et al. Meningiomas: MR and histopathologic features. Radiology 1989;170:857–62 [DOI] [PubMed] [Google Scholar]
- 17.Koeller KK, Smirniotopoulos JG, Jones RV. Primary central nervous system lymphoma: radiologic-pathologic correlation. Radiographics 1997;17:1497–526 [DOI] [PubMed] [Google Scholar]
- 18.Lee YC, Chueng YC, Hsu SW, et al. Idiopathic hypertrophic cranial pachymeningitis: case report with 7 years of imaging follow-up. AJNR Am J Neuroradiol 2003;24:119–23 [PMC free article] [PubMed] [Google Scholar]
- 19.Buccoliero AM, Caldarella A, Santucci M, et al. Plasma cell granuloma: an enigmatic lesion—description of an extensive intracranial case and review of the literature. Arch Pathol Lab Med 2003;127:e220–23 [DOI] [PubMed] [Google Scholar]
- 20.Despeyroux-Ewers M, Catalaa I, Collin L, et al. Inflammatory myofibroblastic tumour of the spinal cord: case report and review of the literature. Neuroradiology 2003;45:812–17. Epub 2003 Sep 27 [DOI] [PubMed] [Google Scholar]
- 21.Rossi S, Giannini F, Cerase A, et al. Uncommon findings in idiopathic hypertrophic cranial pachymeningitis. J Neurol 2004;251:548–55 [DOI] [PubMed] [Google Scholar]
- 22.Wild T, Strotzer M, Volk M, et al. Idiopathic hypertrophic cranial pachymeningitis associated with an orbital pseudotumor. Eur Radiol 1999;9:1401–03 [DOI] [PubMed] [Google Scholar]
- 23.Deprez M, Born J, Hauwaert C, et al. Idiopathic hypertrophic cranial pachymeningitis mimicking multiple meningiomas: case report and review of the literature. Acta Neuropathol 1997;94:385–89 [DOI] [PubMed] [Google Scholar]
- 24.Adler JR, Sheridan W, Kosek J, et al. Pachymeningitis associated with a pulmonary nodule. Neurosurgery 1991;29:283–87 [DOI] [PubMed] [Google Scholar]
- 25.Riku S, Kato S. Idiopathic hypertrophic pachymeningitis. Neuropathology 2003;23:335–44 [DOI] [PubMed] [Google Scholar]
- 26.Roche PH, Figarella-Branger D, Pellet W. Mixed meningeal and brain plasma-cell granuloma: an example of an unusual evolution. Acta Neurochir (Wien) 2004;146:69–72. Epub 2003 Dec 15 [DOI] [PubMed] [Google Scholar]
- 27.Jung TY, Jung S, Lee MC, et al. Hemorrhagic intracranial inflammatory pseudotumor originating from the trigeminal nerve: a case report. J Neurooncol 2006;76:139–42 [DOI] [PubMed] [Google Scholar]