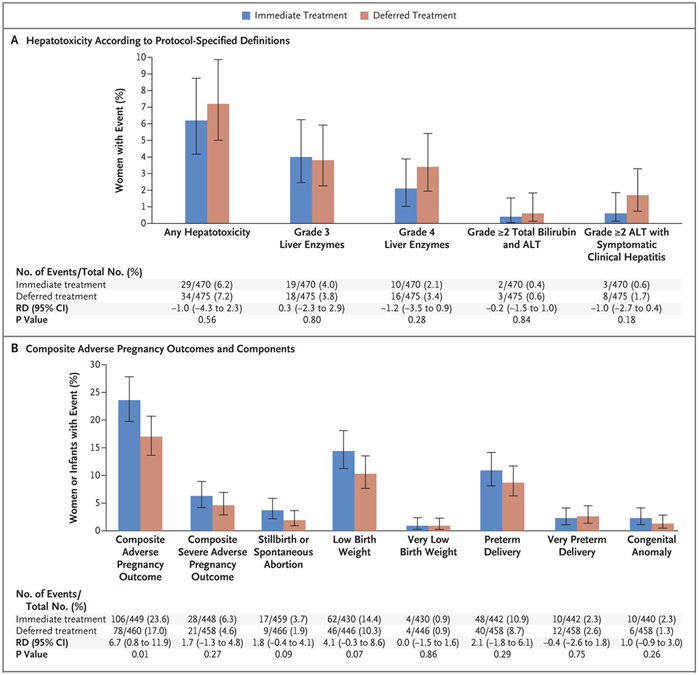
Figure 2. Hepatotoxicity and Adverse Pregnancy Outcomes.
Hepatotoxicity (Panel A) was defined as a grade 3 or higher elevation in liver-enzyme levels (alanine aminotransferase [ALT], aspartate aminotransferase, or total bilirubin), a grade 2 or higher elevation in total bilirubin and ALT levels, or a grade 2 or higher elevation in ALT level with symptomatic clinical hepatitis. Grading of hepatotoxicity was determined on the basis of criteria specified in the protocol. The composite adverse pregnancy outcome (Panel B) was stillbirth (fetal death at 20 weeks of gestation or later) or spontaneous abortion (loss of pregnancy before 20 weeks of gestation), low birth weight (<2500 g) in an infant, preterm delivery (delivery before 37 weeks of gestation, with duration of gestation determined with the use of the Ballard examination when available or by obstetrical estimate), or major congenital anomalies in an infant (defined according to the criteria of the Metropolitan Atlanta Congenital Defects Program of the Centers for Disease Control and Prevention24). The composite severe adverse pregnancy outcome was stillbirth or spontaneous abortion, very low birth weight (<1500 g) in an infant, very preterm delivery (delivery before 34 weeks of gestation), or major congenital anomalies in an infant. The risk difference (RD) is the between-group difference in percentage points.