Figure 5 |. Differential N-glycosylation of human MOR carrying N40D variants.
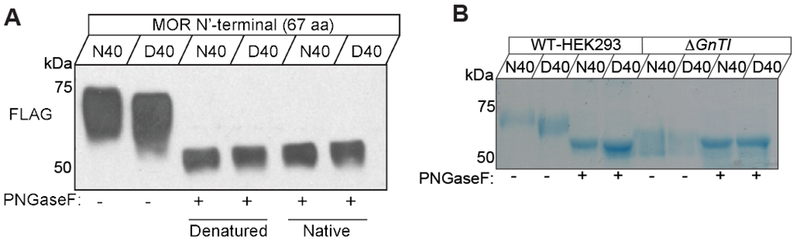
(A) Immunoblot (IB) of recombinant human FLAG-MOR N-terminal region-human Fc (amino acids 1-67) expressed as a soluble entity in HEK 293 cells. Both peptides (N40 and D40) were separated by size on an SDS-PAGE gel and detection was accomplished using an anti-FLAG antibody. Note that the untreated D40 sample (lane 2) is already of lower molecular weight compared to the N40 (lane 1) and after PNGase F digestion (lanes 3-6) both samples show an identical migration pattern, indicating complete removal of the N-linked glycans. PNGase reactions were carried out in two ways. Specifically, either the peptide was denatured prior to enzyme application (lane 3 and 4) or the native peptide was treated with PNGase F (lanes 5 and 6). Glycan removal was equally effective both ways tested. (B) Recombinant human FLAG-MOR N-terminal region-human Fc peptide was purified from the culture media of HEK293S (wild-type) (lanes 1-4) or HEK293S GnT1- (lanes 5-8). SDS-PAGE followed by Coomassie staining revealed that under conditions where glycosylation is defective or abolished N40 and D40 MOR peptide migrate at the same molecular weight (lanes 5 and 6) regardless of PNGase F treatment.
