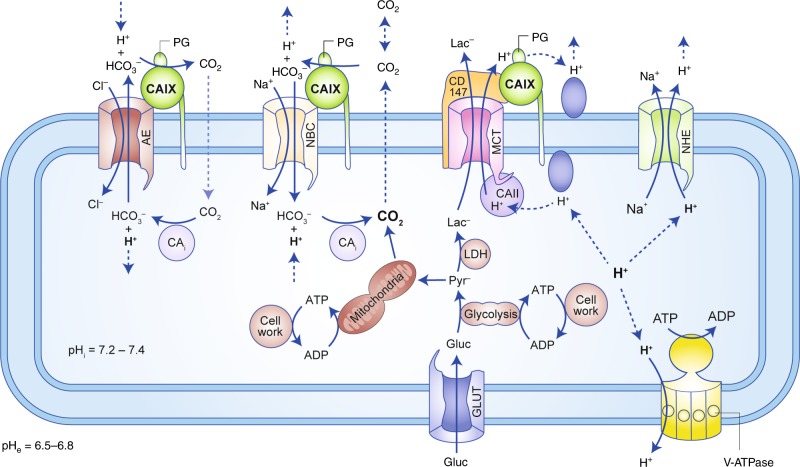
Fig. 1.
Tumour pH is regulated by the concerted interplay between acid/base transporters and carbonic anhydrase. Metabolic acids are produced by glycolysis and mitochondrial respiration. Anaerobic glycolysis yields lactate and H+ that are excreted from the cell by monocarboxylate transporters (MCTs) in a 1:1 stoichiometry. Mitochondrial respiration produces CO2, which is hydrated in the cell, forming HCO3− and H+. CO2 can leave the cell by passive diffusion over the plasma membrane or through gas channels (not shown). Efficient pH regulation requires the function of additional transporters and enzymes, which either export protons from the cell or mediate the reimport of HCO3−. Additional export of H+ can be mediated by the Na+/H+ exchanger 1 (NHE) and by vacuolar H+-ATPase (V-ATPase). CO2 venting is further supported by the catalytic function of the extracellular carbonic anhydrase (CA) isoforms CAIX and CAXII (the latter one is omitted from this cartoon for clarity). Extracellular CAs catalyse the hydration of CO2 to HCO3− and H+ at the membrane. HCO3− can diffuse away from the cell or can be reimported by Na+/HCO3– cotransporters (NBC) to support intracellular buffering. The extracellular HCO3− can either be formed from ‘endogenous' CO2, which is produced by the cell through mitochondrial respiration or titration of HCO3− and H+, or from extracellular CO2, produced from distant sources. Cl−/HCO3− exchangers (AEs) have been suggested to function either as HCO3− importers for pH buffering or HCO3− exporters that extrude HCO3− to load cellular compartments with Cl– during cell migration. Transport activity of many acid/base transporters is facilitated by interaction with intracellular and extracellular CAs. NBC and AE interact with CAII and CAIX that either provide or remove HCO3− to/from the transporter via their catalytic function. MCTs form a protein complex with CAII and CAIX, in which the CAs function as ‘proton antenna’ for the transporter, which mediates the rapid exchange of H+ between transporter pore and the surrounding protonatable residues.