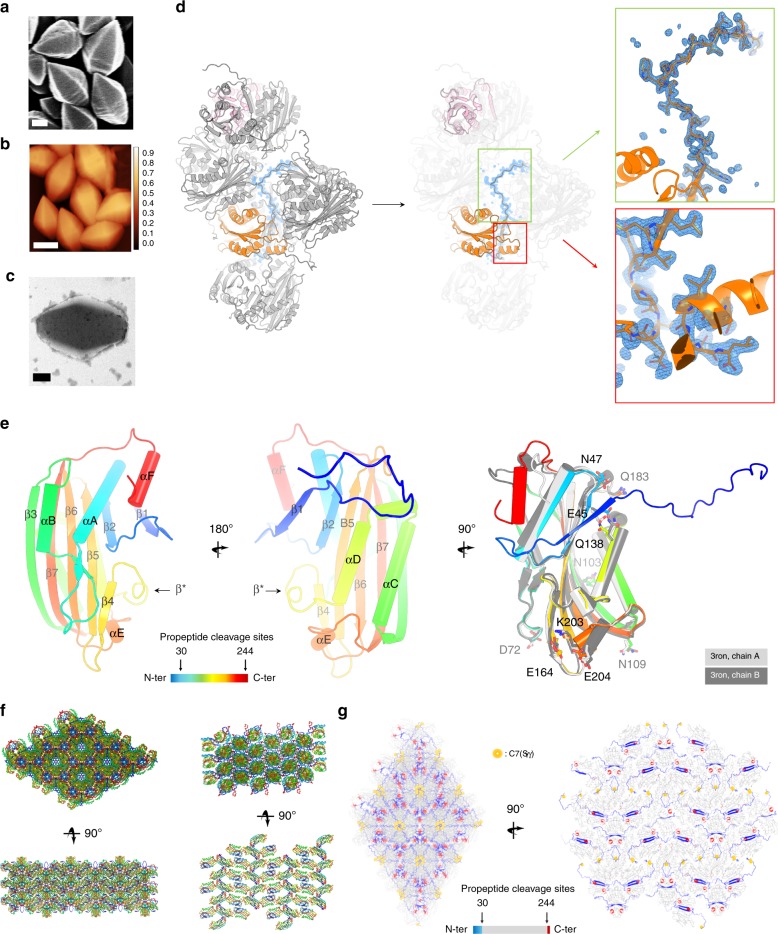
Fig. 1. SFX on in vivo-grown crystals enables determination of the Cyt1Aa protoxin structure.
a–c Cyt1Aa sub-micron-sized crystals were grown in vivo by recombinant expression in Bti-4Q7, and their quality assessed by scanning electron microscopy (SEM; a scale bar = 200 nm), atomic force microscopy (AFM; b scale bar = 500 nm) and transmission electron microscopy (TEM; c scale bar = 200 nm). d Electron density was visible for the propeptides in the initial 2Fo-Fc electron density map displayed at 1 σ. The N-terminal propeptide establishes contact with four symmetry-related molecules. e Tertiary structure of Cyt1Aa. In the right panel, the two chains constitutive of the asymmetric unit of the activated toxin (“3ron [10.2210/pdb3RON/pdb]”) are overlaid on the protoxin structure, with residues displaying different side chain conformations shown as sticks. Secondary structure information is overlaid on the models. f Packing in the natural protoxin crystals grown in vivo (left) and in the crystals of the activated toxin grown in vitro (right). g The N-terminal and C-terminal propeptides scaffold the natural crystals.