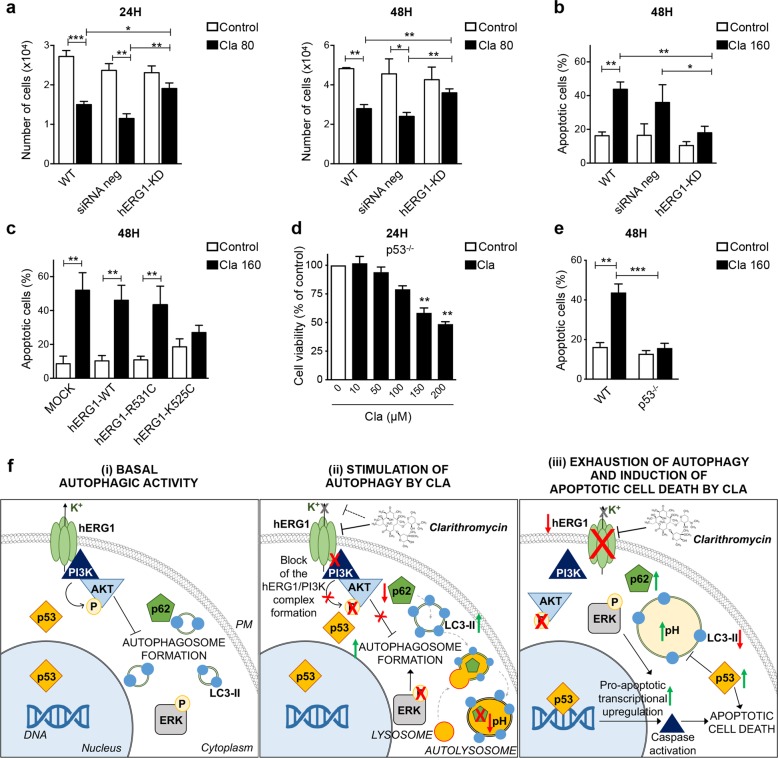
Fig. 6. The pro-apoptotic effect of Clarithromycin is mediated by hERG1 and p53.
a, b HCT116 cells were silenced for hERG1 as in Fig. 3a. Twenty-four hours after siRNA transfection, cells were treated with Cla (80 and 160 µM). After 24 and 48 h of incubation, cells were harvested and cell viability (a) and apoptosis (b) were assessed. Cell viability is expressed as the number of alive, trypan blue-negative, cells. Percentage of apoptotic (early apoptotic plus late apoptotic) cells was determined by the Annexin-V/PI staining (n = 3). c Percentage of apoptotic (early apoptotic plus late apoptotic, determined by the Annexin-V/PI staining) HCT116 cells expressing either hERG1-WT, or hERG1-K525C, or hERG1-R531C, after 48 h of treatment with Cla (160 µM) (n = 3). d Cell viability of HCT116 p53−/− cells after 24 h of treatment with Cla (0–200 µM). Data are reported as percentage of control cells (n = 4, each carried out in triplicate). e Percentage of apoptotic (early apoptotic plus late apoptotic, determined by the Annexin-V/PI staining) HCT116 p53−/− cells treated with Cla (160 µM) for 48 h (n = 3). Statistical significance was assessed with a one-way ANOVA test for (a–f); *P < 0.05; **P < 0.01, and ***P < 0.001. f Schematic diagram summarizing the hypothesized mode of action of Cla in human CRC cells. (i) Basal autophagic activity: hERG1 is highly expressed on the plasma membrane (PM) of HCT116 cells and forms a macromolecular complex with the p85 regulatory subunit of PI3K. The hERG1/PI3K complex leads to Akt phosphorylation, which regulates autophagy. (ii) Stimulation of autophagy by Cla: Cla inhibits the hERG1/PI3K complex formation, which leads to reduced Akt and ERK1/2 phosphorylation, and increases autophagic activity. This effect is correlated to an increase in LC3-II conversion and accumulation, a reduction of p62/SQSTM1 and the accumulation of autolysosomes. (iii) Exhaustion of autophagy and induction of apoptotic cell death by Cla: prolonged treatment with Cla leads to hERG1 degradation, and an impairment of the autophagic process, characterized by increased vacuole size and intra-vacuolar alkalinization. As a consequence, expression of p62/SQSTM1 and phosphoERK1/2 recovers and LC3-II accumulation decreases. These effects are also correlated to a late recovery of p53, responsible for caspases activation and apoptotic cell death.