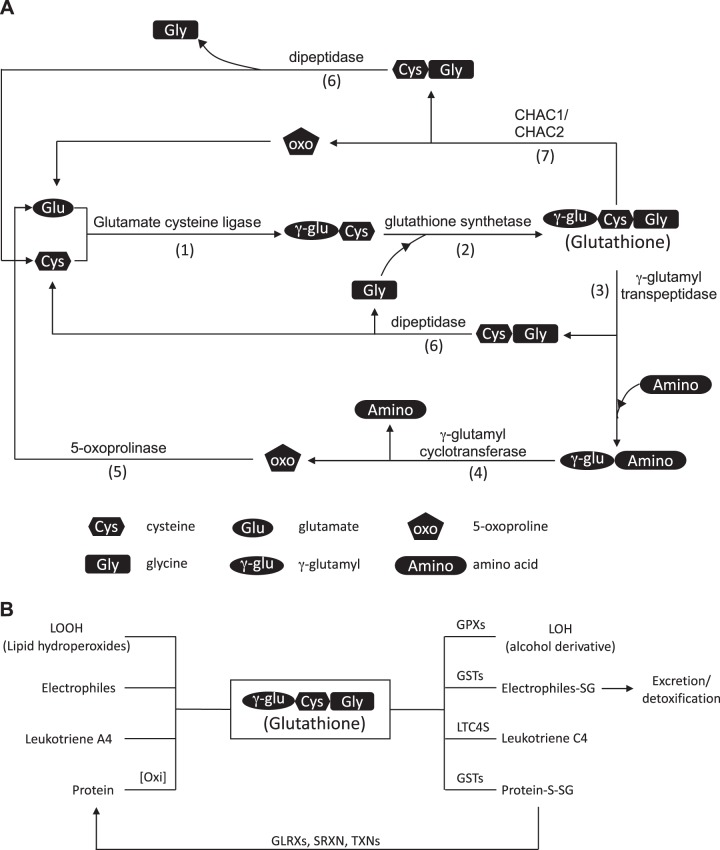
Fig. 1.
A: reduced glutathione (GSH) metabolism. Glutamate cysteine ligase (GCL), composed of a catalytic subunit GCLC and a regulatory unit GCLM, catalyzes the formation of γ-glutamylcysteine (1). Glutathione synthetase (GS) then completes the synthesis of GSH by adding glycine to γ-glutamylcysteine (2). GSH is broken down to cysteinylglycine by γ-glutamyl transpeptidase (GGT) (3). The γ-glutamyl group can be recycled through multiple steps where it is converted to 5-oxoproline by γ-glutamyl cyclotransferase (GGCT) (4) before it is converted back to glutamate by 5-oxoprolinase (OPLAH) (5). Cysteinylglycine is broken down into cysteine and glycine by dipeptidase (DP), where they are recycled or are shunted into other metabolic pathways (6). Alternatively, glutathione-specific γ-glutamylcyclotransferase (ChaC)1 and ChaC2 break down GSH into cysteinylglycine and 5-oxoproline (7). B: GSH utilization. GSH is conjugated to 1) oxidized lipid (LOOH) and through the catalytic reaction of glutathione peroxidase (GPXs) reduces oxidized lipid to its reduced form (LOH); 2) electrophiles and other xenobiotics through the catalytic reaction of glutathione S-transferases (GSTs) in phase II drug metabolism; 3) leukotriene A4 through the action of leukotriene C4 synthase (LTC4S), forming leukotriene C4; and 4) proteins to prevent protein cysteines from undergoing irreversible oxidation. GSH is also used in the regeneration of the sulfhydryl group to its reduced state through the catalytic activity of glutaredoxins.