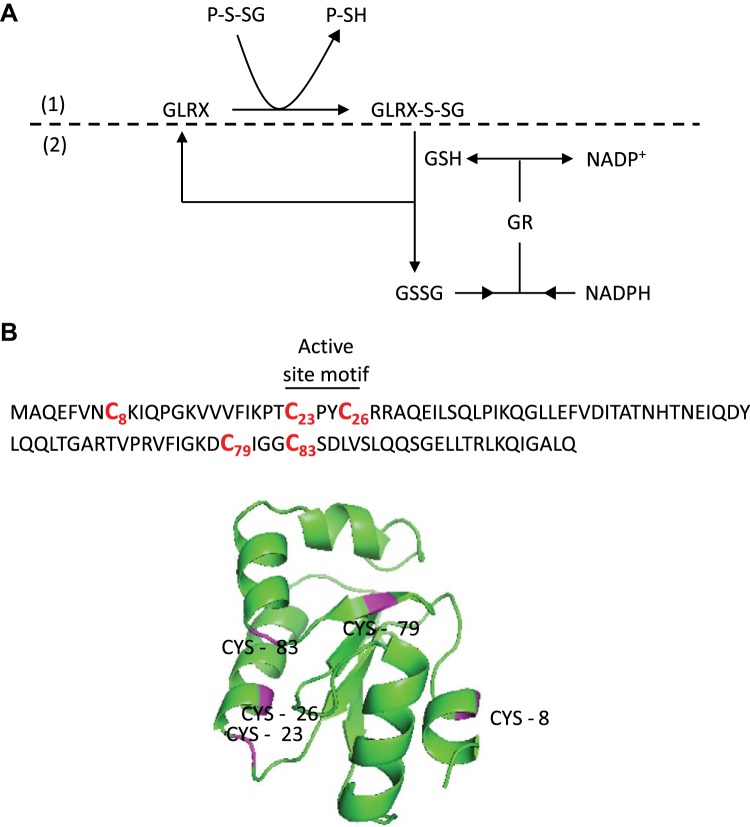
Fig. 3.
Human glutaredoxin-1 (GLRX) primary sequence, 3D structure, and deglutathionylation mechanism. A: GLRX catalyzes deglutathionylation. GLRX deglutathionylates proteins and is glutathionylated in the process (1). GLRX is regenerated by consuming GSH, which is in turn regenerated through the reduction of oxidized glutathione (GSSG) by glutathione reductase (GR) with NADPH as the final electron donor (2). B: the 5 cysteines that comprise human GLRX are indicated. Note that only Cys23 is required for deglutathionylation activity. Cys8, Cys79, and Cys83 have been linked to oxidative inactivation of GLRX.