Abstract
Tobacco smoking has been identified as a risk factor in the progression of chronic kidney disease (CKD). In previous studies, we showed that nicotine induces cyclooxygenase (COX)-2 expression in vivo and in vitro and that the administration of nicotine in vivo worsens the severity of renal injury in a model of subtotal renal ablation. In the present study, we tested the role of COX-2-derived prostaglandins on the deleterious effects of nicotine in CKD. Sham and 5/6 nephrectomy (5/6Nx) rats received tap water or nicotine (100 μg/mL) in the drinking water for 12 wk. Additional groups also systemically received the COX-2 inhibitor NS-398 (1.5 mg·kg−1·day−1 via osmotic minipump). The administration of nicotine worsened renal injury and proteinuria in 5/6Nx rats and increased proteinuria in sham rats. 5/6Nx rats had increased cortical production of the prostaglandins PGE2, PGI2, PGD2, and PGF2α and of thromboxane A2. In these rats, nicotine reduced the production of all prostaglandins examined except thromboxane A2. Treatment with the COX-2 inhibitor NS-398 resulted in complete inhibition of all prostaglandins studied and ameliorated renal injury and proteinuria in 5/6Nx rats on nicotine but not in 5/6 Nx rats on tap water. Nicotine also reduced the expression of megalin in all groups examined, and this was partially prevented by COX-2 inhibition. In the present study, we showed that in CKD, nicotine worsens renal injury at least in part by producing an imbalance in the production of prostaglandins. This imbalance in the production of prostaglandins likely plays a role in the deleterious effects of smoking on the progression of CKD.
Keywords: chronic kidney disease, cyclooxygenase, nicotine, prostaglandins, smoking
INTRODUCTION
Tobacco smoking is the most important cause of preventable morbidity and mortality in the United States because it increases the risk for death because of accelerated atherosclerosis, cardiovascular disease, emphysema, and different types of cancer (24, 55). In addition, tobacco smoking is a well-established independent risk factor in the progression of chronic kidney disease (CKD) of different etiologies, including hypertension and diabetes (1, 23, 29, 40, 41, 47).
However, the mechanisms by which tobacco smoking accelerates the progression of CKD are not completely understood. The gas phase of tobacco smoke is composed of more than 5,000 different compounds, many of them biologically active (14, 25). In addition to short-lived reactive oxygen species, tobacco smoke contains high concentrations of stable biologically active compounds such as nicotine, which is not only responsible for the addictive properties of tobacco smoking but in addition is implicated in the pathogenesis of tobacco-induced atherosclerosis (15) and pulmonary fibrosis (46). Moreover, as we have previously shown, nicotine worsens glomerular injury in a rat model of acute nephritis (19), promotes extracellular matrix deposition in a mouse model of diabetic nephropathy (17), and increases the severity of injury in a rat model of subtotal renal ablation (21).
Nicotine mediates its effects via the activation of muscle and neuronal nicotinic acetylcholine receptors (nAChRs) (2). nAChRs are a family of transmembrane ligand-gated ion channels consisting of five subunits that result from the combinatorial assembly of different nAChR subunits. To date, seven α-like subunits (α2–α7, α9, and α10) and three non-α subunits have been identified (β2–β4). Receptor pentamers can be constructed from various combinations of α and β (2). nAChRs are also expressed in non-neuronal cells, including bronchial epithelial cells, endothelial cells, vascular smooth muscle cells, and human mesangial cells (12, 16, 21, 33, 34, 53).
We have recently demonstrated the expression of α7-nAchR in the rat renal cortex and demonstrated that pharmacological blockade of this subunit prevents the effects of nicotine on renal injury in a rat model of 5/6 nephrectomy (5/6Nx), a well-characterized and validated model of CKD that closely mimics CKD in humans (44). We have also shown that the administration of nicotine increases the expression of cyclooxygenase (COX)-2 in rats with anti-Thy-1 and, in vitro, increases the expression of COX-2 in rat mesangial cells (19). Moreover COX-2 inhibition prevents the effects of nicotine on mesangial cell proliferation (19, 20).
In the present study, we hypothesized that COX-2-derived prostaglandins mediate, at least in part, the deleterious effects of the chronic administration of nicotine in the progression of CKD. To test this hypothesis, we used 5/6Nx as a model for CKD as previously shown (44).
METHODS
Animal protocol.
Male 8-wk-old Sprague-Dawley rats (Charles River, Boston, MA) were kept under controlled conditions of light, humidity, and temperature. Animals were housed in facilities accredited by the American Association for Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee of the University of Alabama at Birmingham approved these experiments. Rats were subjected to either sham surgery or 5/6Nx as previously described (44). They were divided into the following groups: sham-operated rats that drank tap water (Sham group; n = 8), sham rats that received nicotine (100 μg/mL) in the drinking water (ShamNic group; n = 8), sham rats that received nicotine (100 μg/mL) in the drinking water and the COX-2 inhibitor NS-398 (1.5 mg·kg−1·day−1) via osmotic minipump (ShamNicNS group; n = 8), rats with 5/6Nx that received tap water (5/6Nx group; n = 7), rats with 5/6Nx that received nicotine (100 μg/mL) in the drinking water (5) (5/6NxNic group; n = 8), rats with 5/6Nx that received nicotine (100 μg/mL) in the drinking water and NS-398 (15) (1.5 mg·kg−1·day−1) via osmotic minipump (5/6NxNicNS group, n = 8), and rats with 5/6Nx on tap water and that received NS-398 (1.5 mg·kg−1·day−1) via osmotic minipump (5/6NxNS group; n = 8). Rats remained in their treatment groups for 12 wk after surgery and were euthanized under deep anesthesia by exsanguination by cardiac puncture followed by cervical dislocation. Kidneys were collected for Western blot analysis and histology, and blood was collected for serum cotinine and creatinine measurements.
Biweekly blood pressure measurements were carried out in all groups by the tail-cuff method (CODA, Kent Scientific, CT). Simultaneous urine collections were also performed biweekly in all groups in metabolism cages. Animals were housed in facilities accredited by the American Association for Accreditation of Laboratory Animal Care, and the study was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Nephrectomies.
5/6Nx was performed in two steps under general anesthesia (isofluorane: 2.5%) using the full sterile technique as described below and as previously described (44). First, using a retroperitoneal approach, a subtotal nephrectomy was performed in which both poles of the left kidney were surgically excised, and the stumps were ligated. Gel-Foam was used for hemostasis. A total right nephrectomy was then performed after ligation of the renal vessels and the ureter 1 wk after the first procedure.
Proteinuria.
Proteinuria was measured by the Lowry method and adjusted by urinary creatinine (mass spectrometry). Urinary albumin concentrations were measured using a Rat Albumin ELISA Quantitation kit from Bethyl (Montgomery, TX) and were adjusted for urinary creatinine measured by mass spectrometry.
Serum creatinine.
Serum creatinine was determined by mass spectrometry at the O’Brien Kidney Center Core Facilities at the University of Alabama at Birmingham.
Western blot analysis.
Western blots were performed as previously described (20). Briefly, 100 mg of kidney cortex was homogenized in 300 μL of homogenization buffer [20 nmol/L Tris·HCl (pH 7.4), 140 mmol/L NaCl, 10 mmol/L Na pyrophosphate, 10 mmol/L Na fluoride, 2 mmol/L Na orthovanadate, 3 mmol/L EDTA, and 10% glycerol]. Protease inhibitor cocktail (P8340, Sigma) was added to the homogenization buffer before use. The resulting lysates were centrifugated for 20 min at 13,200 revolutions/min at 4°C. The supernatants were collected, and protein concentration was quantified by the Lowry method.
For immunoblot analysis, 15–20 μL of homogenate were separated by SDS-PAGE (10–15% acrylamide gel) and transferred to a nitrocellulose membrane (0.2 μm, Bio-Rad). The blots were incubated with antibodies against fibronectin (Sigma, St. Louis, MO) and megalin (Abcam, Cambridge, MA). β-actin (Sigma) was used to control for loading. The blots were washed and all incubated with anti-rabbit (Jackson, West Grove, PA), whereas β-actin was incubated with anti-mouse (Jackson, West Grove, PA), and the signal was detected by luminol chemoluminescence (Millipore, Billerica, MA).
Immunofluorescence.
Formalin-fixed, paraffin-embedded rat kidney sections (5 μm) were deparaffinized, and antigen retrieval was performed on all sections using Vector Laboratories’ antigen retrieval solution. Sections used to identify specific tubules were incubated for 15 min each with avidin blocking reagent and biotin blocking reagent. Sections were blocked with PBS + 1% BSA and 5% goat serum for 1 h at room temperature and then incubated overnight (at 4°C in a humidified chamber) with primary antibody to fibronectin (Sigma) and megalin (Abcam) at a 1:200 dilution in blocking buffer. Sections were then washed three times for 5 min with PBS and incubated (1 h at room temperature) with the secondary antibody Alexa Fluor 488-labeled goat anti-rabbit (Molecular Probes, Portland, OR) at 1:400 dilution in blocking buffer. Sections were then washed three times for 5 min with PBS and mounted with coverslips. Image acquisition was performed on a Leica DM6000 epifluorescence microscope (Leica Microsystems, Bannockburn, IL) with a Hamamatsu ORCA ER cooled charge-coupled device camera and SimplePCI software (Compix, Cranberry Township, PA). Images were adjusted appropriately to remove background fluorescence. Identical sections used as controls were processed without either primary antibody or secondary antibody present during identical incubation conditions.
Immunoshistochemistry.
Formalin-fixed, paraffin-embedded kidney sections (5 μm) were deparaffinized and antigen retrieval performed on all sections using Vector Laboratories’ antigen retrieval solution. The avidin-biotin-peroxidase immunohistochemical technique (ABC kit, Vector Laboratories) was used to detect nitrotyrosine expression using monoclonal mouse antibody (Abcam).
Glomerular injury score.
Glomerular injury score was measured in periodic acid-Schiff-stained kidney slides by one of the co-authors (H. Fatima), an experienced pathologist purposely blinded to the experimental conditions and using a 0+ to 4+ scale (43). All glomeruli available in each slide were analyzed, and the data are expressed as the percentage of glomeruli injured at every level.
Serum cotinine.
Serum concentrations of cotinine, a stable metabolite of nicotine, were determined by using a mouse/rat cotinine ELISA kit (Calbiotech, San Diego, CA) following the manufacturer’s instructions.
Isoprostanes.
Urinary isoprostanes were measured as markers of oxidative stress. They were determined by using an OxiSelect 8-iso-Prostaglandin F2α ELISA kit (Cell Biolabs, San Diego, CA) following the manufacturer’s instructions.
Tissue prostaglandins.
Prostaglandins PGE2, PGD2, the stable PGI2 metabolite PG 6-keto-PGF1α, the stable thromboxane A2 metabolite of thromboxane B2, and PGF2α were measured by HPLC as previously described in kidney cortex and medulla homogenates (6).
Statistical analysis.
All data were expressed as means ± SE. Statistical analysis was performed by ANOVA (Statistix 8 Analytical Software, Tallahassee, FL). Significance was considered when P < 0.05 using the least significant difference all-pairwise comparisons test.
RESULTS
Nicotine worsens glomerular injury in rats with CKD.
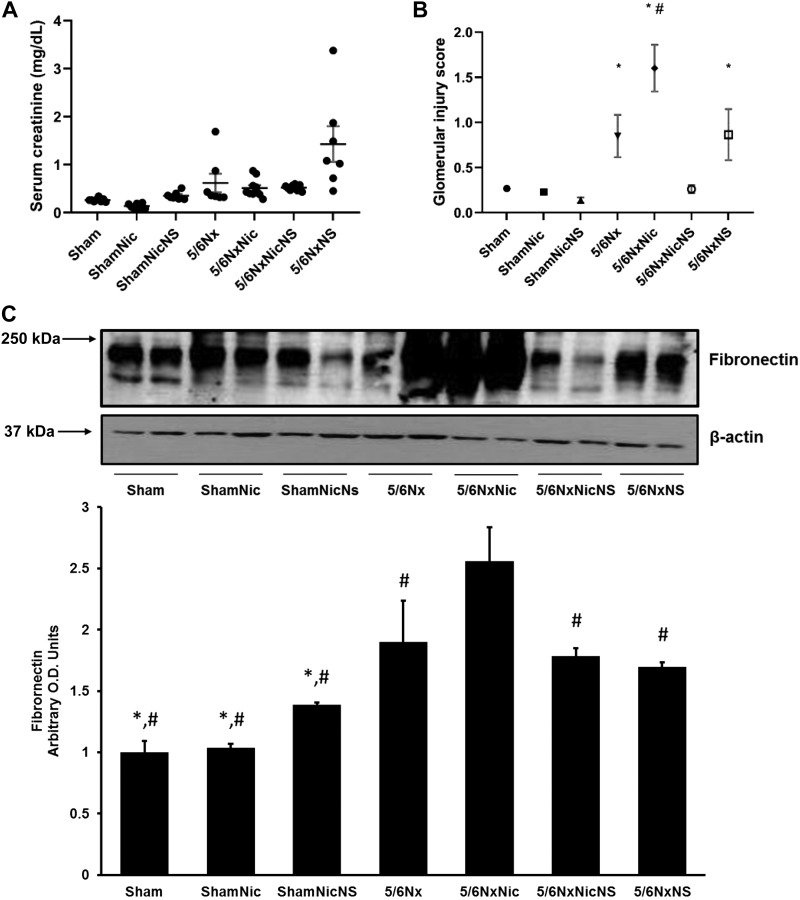
The administration of nicotine resulted in increased levels of cotinine, the stable nicotine metabolite, similar to those found in the plasma of active smokers (52) (ShamNic group: 38.7 ± 0.1 ng/mL, ShamNicNs group: 54.8 ± 0.4 ng/mL, NxNic group: 74.7 ± 3.8 ng/mL, and NxNicNs group: 57.0 ± 0.3 ng/mL). As expected, levels of cotinine in animals on tap water were below the level of detection (not shown). Although the administration of nicotine did not result in significant changes in serum creatinine (Fig. 1A), it resulted in significant worsening of renal injury as assessed by glomerular injury score in 5/6Nx rats but had no effect in sham rats (Fig. 1B). The administration of the COX-2 inhibitor NS-398 resulted in significant reductions in renal injury in 5/6Nx rats on nicotine but not in 5/6Nx rats on tap water (Fig. 1B). These changes in glomerular injury score were accompanied by concomitant changes in fibronectin expression as assessed by Western blot analysis (Fig. 1C). Sham rats that received nicotine had higher systolic blood pressure at 12 wk compared with sham rats on tap water (Sham group: 128 ± 5 mmHg and ShamNic group: 152 ± 5 mmHg, P < 0.05 vs. the Sham group). These effects were ameliorated by COX-2 inhibition (ShamNicNs group: 137 ± 9 mmHg, P = not significant vs. the Sham group). Rats with 5/6Nx either on tap water or that received nicotine had higher blood pressures compared with sham rats (5/6Nx group: 161 ± 8 mmHg and 5/6NxNic group: 159 ± 8 mmHg, P < 0.05 vs. the sham group). The administration of NS resulted in nonsignificant reductions in blood pressure compared with 5/6Nx or 5/6NxNic groups (5/6NxNicNS group: 145 ± 9 mmHg and 5/6NxNS group: 154 ± 11 mmHg, P = not significant).
Fig. 1.
A: serum creatinine (in mg/dL) measured by mass spectrometry showed no significant difference between the groups (P = not significant). B: glomerular injury score showed significant injury in 5/6 nephrectomy (5/6Nx) rats that was worsened by nicotine (5/6NxNic) and improved by cyclooxygenase (COX)-2 inhibition. *P < 0.05 vs. sham-operated rats (Sham group), sham rats that received nicotine in the drinking water (ShamNic group), and sham rats that received nicotine and the COX-2 inhibitor NS-398 (ShamNicNS group). #P < 0.05 vs. 5/6Nx rats that received nicotine and NS-398 (5/6NicNS group). C: representative Western blot and quantitation of fibronectin, which showed increase in fibronectin in the 5/6Nx group treated with nicotine that was attenuated by COX-2 inhibition (*P < 0.05 vs. 5/6Nx; #P < 0.05 vs. 5/6NxNic). O.D., optical density.
COX-2 inhibition mitigates nicotine-induced proteinuria.
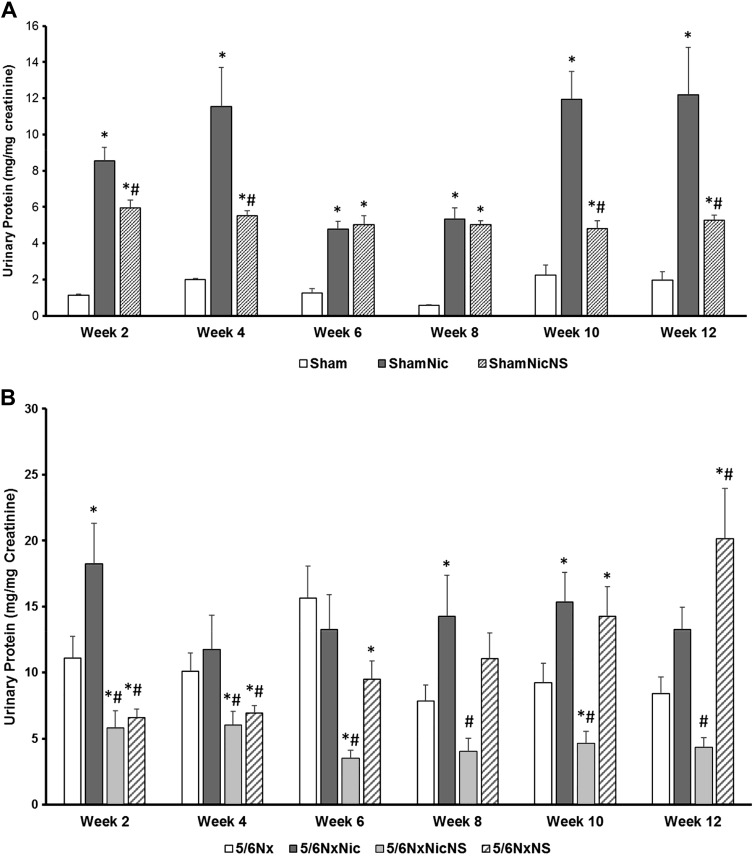
The administration of nicotine induced proteinuria in sham rats and worsened proteinuria in 5/6Nx rats, which was evident as early as 2 wk after administration. Although there was no significant progression of the degree of proteinuria, proteinuria persisted through the 12-wk experimental period. Administration of the COX-2 inhibitor NS-398 significantly decreased proteinuria in both sham and 5/6Nx rats (Fig. 2).
Fig. 2.
Proteinuria measured every 2 wk throughout the duration of the study. A: sham rats had increased proteinuria when given nicotine in the drinking water, which was partially prevented by cyclooxygenase (COX)-2 inhibition [*P < 0.05 vs. the Sham group; #P < 0.05 vs. sham-operated rats given nicotine (ShamNic group)]. B: the administration of nicotine worsened proteinuria in 5/6 nephrectomy (5/6Nx) rats but was prevented by COX-2 inhibition. In rats with 5/6Nx and tap water, COX-2 inhibition worsened proteinuria during the last 4 wk of the study [*P < 0.05 vs. the 5/6Nx group; #P < 0.05 vs. 5/6Nx rats given nicotine (5/6NxNic group)]. 5/6NxNicNS, 5/6Nx rats given nicotine and NS-398; 5/6/NxNS, 5/6Nx rats given NS-398; ShamNicNS, sham-operated rats given nicotine and NS-398.
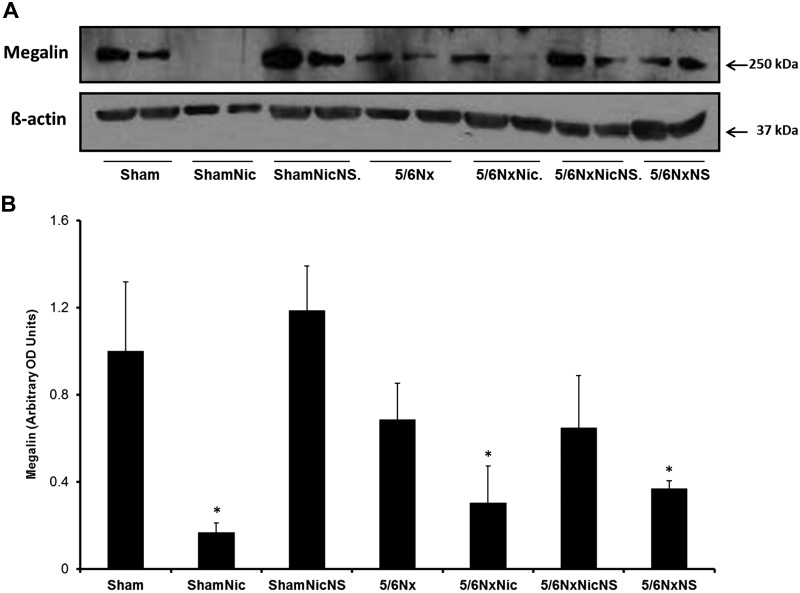
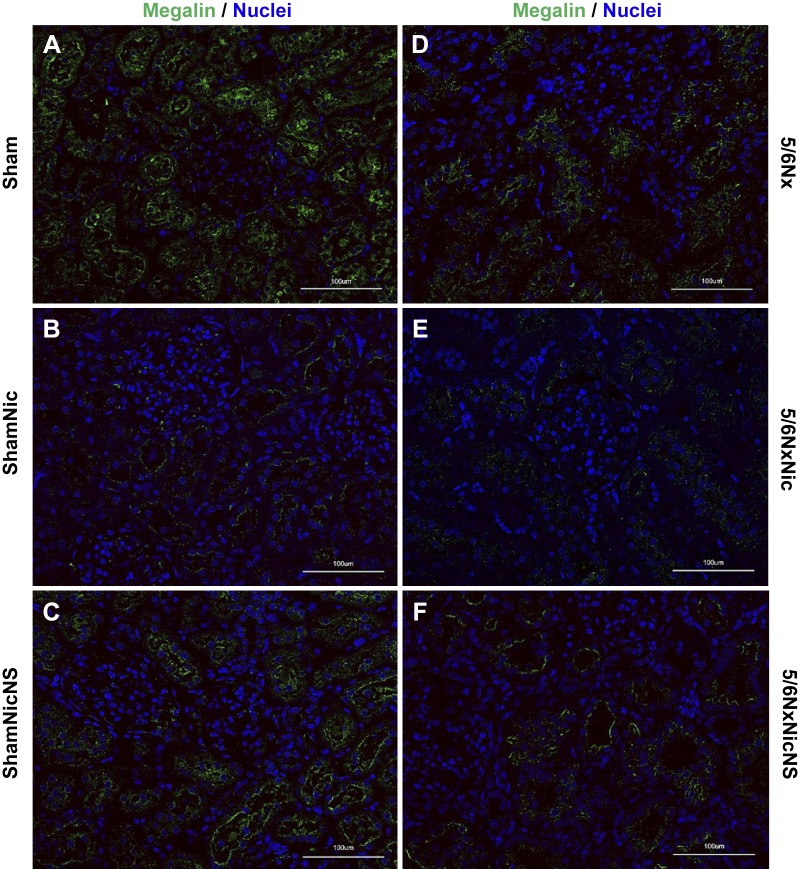
Given the effects of nicotine on proteinuria in sham rats without obvious glomerular injury, we examined the effects of nicotine on megalin expression, a molecule that is expressed in the proximal tubule and plays a key role in tubular reabsorption of proteins (4, 8). Western blot analysis of the tissue lysate showed that the administration of nicotine resulted in a large reduction in megalin expression in sham rats that was reverted by the administration of the COX-2 inhibitor NS-398. Rats with 5/6Nx also had significant reductions in megalin expression that were further enhanced by nicotine and partially prevented by COX-2 inhibition. In contrast, COX-2 inhibition in 5/6Nx rats had only a small effect on megalin expression (Fig. 3). These findings were consistent with immunofluorescence staining for megalin (Fig. 4).
Fig. 3.
Expression of megalin by Western blot showed a significant decrease in expression with nicotine treatment in both sham and 5/6 nephrectomy (5/6Nx) groups. This effect was reversed by treatment with cyclooxygenase-2 inhibitor. A: representative Western blot for megalin and actin, which was used as a loading control. B: densitometry analysis for megalin after adjustment for actin. (*P < 0.05 vs. the Sham group). 5/6NxNic, 5/6Nx rats given nicotine; 5/6NxNicNS, 5/6Nx rats given nicotine and NS-398; 5/6/NxNS, 5/6Nx rats given NS-398; ShamNic, sham rats given nicotine; ShamNicNS, sham-operated rats given nicotine and NS-398; OD, optical density.
Fig. 4.
Megalin expression in sham rats (Sham group; A), sham rats given nicotine (ShamNic group; B), sham rats given nicotine and NS-398 (ShamNicNS group; C), 5/6 nephrectomy rats (5/6Nx group; D), 5/6Nx rats given nicotine (5/6NxNic group; E), and 5/6Nx rats given nicotine and NS-398 (5/6NxNicNS group; F) as assessed by immunofluorescence, which showed decreased in megalin staining in the proximal tubules (green) with nicotine treatment in both sham (B) and 5/6Nx groups (E). This effect was reversed when treated with cyclooxygenase-2 inhibition (C and F).
Prostaglandins play a role in nicotine-mediated glomerular injury.
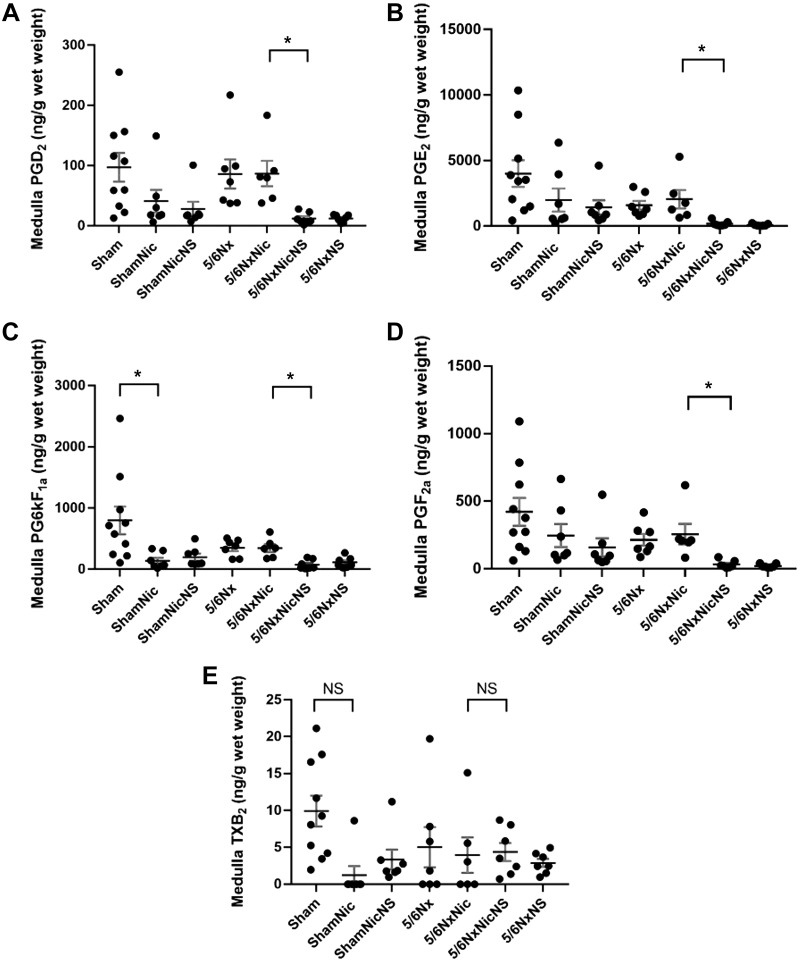
To determine the effects of nicotine on the production of prostaglandins, we measured the cortical and medullary production of prostaglandins using a LC-MS approach. The administration of nicotine to sham animals resulted in significant increases in the cortical production of all the prostaglandins examined, which was inhibited by the administration of the COX-2 inhibitor NS-398 (Fig. 5). In rats with 5/6Nx, as previously reported by others (13), we also observed significant increases in the production of all prostaglandins examined. The administration of nicotine to 5/6Nx rats resulted in significant reductions in the production of all prostaglandins studied except TXA2. The administration of NS-398 resulted in complete inhibition the production of all prostaglandins examined, suggesting that COX-2 was the main source for these prostaglandins. In contrast, the production of prostaglandins in the renal medulla was reduced by the administration of nicotine in sham rats but was not statistically significant except for PGI2 and was not significantly modified by 5/6Nx but was inhibited by NS-398 (Fig. 6). These results suggest that COX-2-derived prostaglandins in the renal cortex play an important role as mediators of injury in rats with 5/6Nx that received nicotine in the drinking water.
Fig. 5.
Cortical production of prostaglandins showing increased production of all prostaglandins [A: PGD2, B: PGE2, C: PG6kF1a, D: PGF2a, and E: thromboxane B2 (TXB2)] examined in 5/6 nephrectomy (5/6Nx) rats; all prostaglandins were reduced by nicotine except for TXB2. Cyclooxygenase-2 inhibition reduced all prostaglandins measured (*P < 0.05). 5/6NxNic, 5/6Nx rats given nicotine; 5/6NxNicNS, 5/6Nx rats given nicotine and NS-398; 5/6NxNS, 5/6Nx rats given NS-398; ShamNic, sham-operated rats given nicotine; ShamNicNS, sham-operated rats given nicotine and NS-398.
Fig. 6.
Medullary production of prostaglandins [A: PGD2, B: PGE2, C: PG6kF1a, D: PGF2a, and E: thromboxane B2 (TXB2)] showing a nonsignificant reduction in the production of prostaglandins except PGI2 in sham rats on nicotine (ShamNic group). No significant change in rats with 5/6 nephrectomy (5/6Nx), and there was reduction in their prostaglandin production by cyclooxygenase-2 inhibition (*P < 0.05). 5/6NxNic, 5/6Nx rats given nicotine; 5/6NxNicNS, 5/6Nx rats given nicotine and NS-398; 5/6NxNS, 5/6Nx rats given NS-398; ShamNicNS, sham-operated rats given nicotine and NS-398. NS, not significant.
COX-2 inhibition did not result in oxidative stress reduction.
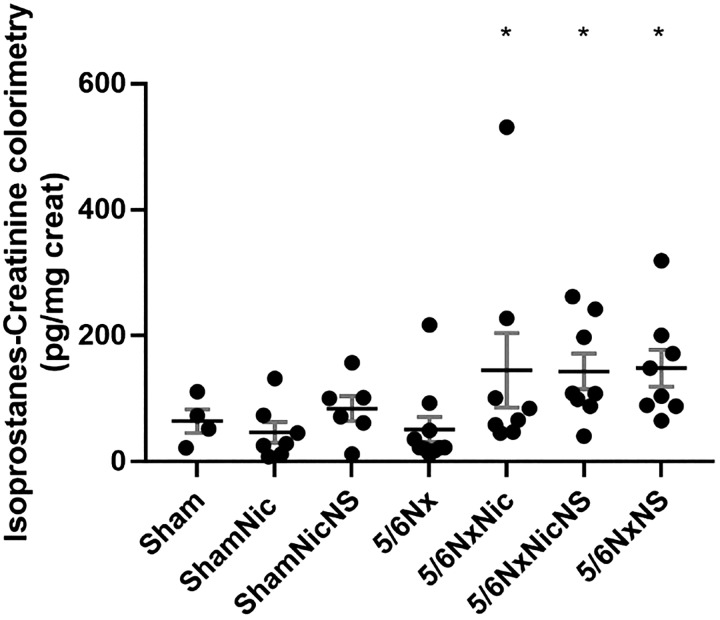
The administration of nicotine increases oxidative stress in a model of CKD, as previously demonstrated (44). The administration of nicotine increased the urinary excretion of isoprostanes (Fig. 7) and nitrotyrosine expression (Fig. 8) in 5/6Nx rats, but this was not affected by administration of the COX-2 inhibitor NS-398.
Fig. 7.
The production of isoprostanes was significantly increased in 5/6 nephrectomy (5/6Nx) rats on nicotine but was not affected by cyclooxygenase-2 (COX-2) inhibition. Rats with 5/6Nx that received COX-2 inhibitor also had increased isoprostanes compared with 5/6Nx rats on tap water (*P < 0.05 vs. the 5/6Nx group). 5/6NxNic, 5/6Nx rats given nicotine; 5/6NxNicNS, 5/6Nx rats given nicotine and NS-398; 5/6NxNS, 5/6Nx rats given NS-398; ShamNic, sham-operated rats given nicotine; ShamNicNS, sham-operated rats given nicotine and NS-398.
Fig. 8.
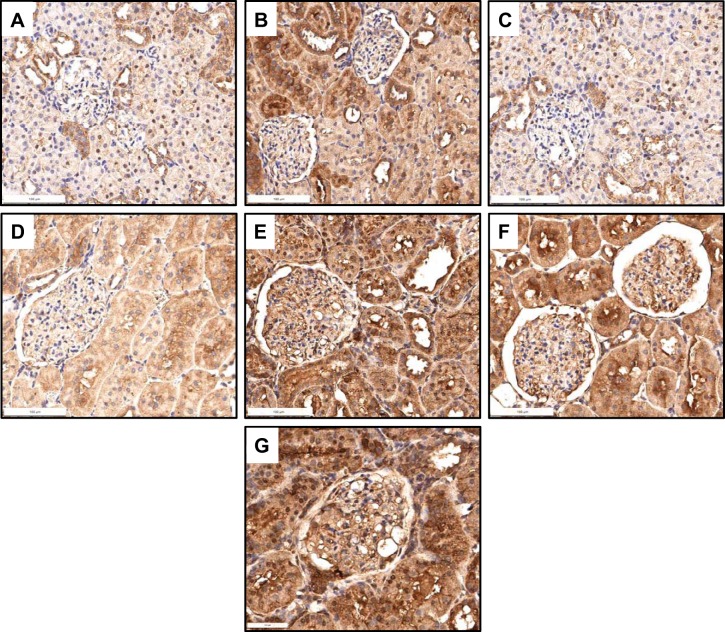
Compared with sham rats (A), the expression of nitrotyrosine was modestly increased in control rats that received nicotine (B), and the expression was normalized by cyclooxygenase-2 (COX-2) inhibition (C). Compared with rats with 5/6 nephrectomy (5/6Nx) and on tap water (D), the administration of nicotine to 5/6Nx rats resulted in a significant increase in nitrotyrosine expression (E) that was not affected by COX-2 inhibition (F). Rats with 5/6Nx that received NS-398 (G) also had a significant increase in nitrotyrosine expression.
DISCUSSION
In the present study and as we have previously proven (44), we showed that nicotine worsened glomerular injury (Fig. 1) and proteinuria (Fig. 2) in a model of 5/6Nx. We did not observe major changes in serum creatinine, which often lags behind other markers of injury, such as glomerular injury, proteinuria, and fibrosis. This phenomenon is often seen in clinical and preclinical models since a significant nephron reserve must be depleted to cause an increase in serum creatinine (5, 54). In our study, we therefore focused on evaluating glomerular injury, proteinuria, and activation of profibrotic pathways. Extracellular matrix proteins such as fibronectin play a key role in kidney fibrosis and progression of CKD (31, 32, 35, 51), and fibronectin has been shown to be highly expressed in the kidneys of patients with CKD of different etiologies (51). Our previous study in vitro demonstrated that nicotine stimulates mesangial cell proliferation and fibronectin production (28), and in vivo we have shown that nicotine increases fibronectin expression in 5/6Nx rats (44). We have demonstrated that nicotine increases the expression of COX-2 in rat mesangial cells (19) and that COX-2 inhibition prevents the effects of nicotine on mesangial cell proliferation and extracellular matrix production (19, 20). In the present study, we now demonstrate that these effects of nicotine on fibronectin expression, glomerular injury, and proteinuria were mitigated by the COX-2 inhibitor NS-398, indicating a key role for COX-2-derived prostaglandins on these effects. Notably rats with 5/6Nx that received nicotine and NS-398 had a drastic reduction in the severity of renal injury and proteinuria, which we speculate may be due to the inhibition of deleterious prostaglandins, such as TXA2, and perhaps other still-to-be-identified effects of nicotine that could result in the activation of potentially beneficial pathways.
It is well known that increased proteinuria is linked to the progression of CKD (9, 18, 42, 48). In our study, we also found that nicotine significantly increased proteinuria in both sham and 5/6Nx rats without inducing any obvious renal injury in sham rats. Proteins filtered by the glomeruli are reabsorbed by two key transporters, namely, megalin and cubilin (3, 38), and downregulation of these proteins results in proteinuria in both experimental models and humans (30, 39, 49). To address the potential mechanisms involved, we measured the expression of megalin and determined that nicotine reduces the expression of megalin in sham rats as well as 5/6Nx rats. When the COX-2 inhibitor NS-398 was given along with nicotine, the expression of megalin was restored to baseline, suggesting that these effects were mediated via COX-2. This finding was confirmed by both quantifying the protein expression in the tissue lysate by Western blot analysis (Fig. 3) and by immunofluorescence staining of the tissues (Fig. 4).
We have previously shown that COX-2 is upregulated in the cortex of hypertensive Dahl salt-sensitive rats (22) and linked to increased urinary excretion of PGE2 (22). We have also shown that angiotensin II can increase glomerular COX-2 expression and activity and that COX-2 inhibition reduces angiotensin II-induced mesangial proliferation and hypertrophy (20). Prostaglandins play a complex dual role in inflammation and exert different effects in different organs (45, 50). Prostaglandins in the kidney have been shown to play a role in vasoconstriction and ischemia, and they are regulated by angiotensin II and can have either beneficial or deleterious effects based on the type of prostaglandin and specific pathological situation (10, 11, 20, 22, 45, 50). The right balance between each of the prostaglandins plays a key role in maintaining normal homeostasis in the kidney. In our present study, we showed a significant increase in the cortical expression of PGD2, PGE2, PGF2α, PGI2, and TXA2 after 5/6Nx, as others have previously shown (13). Nicotine increased the cortical production of all prostaglandins investigated in sham rats but reduced their production, except in the case of TXA2, in 5/6Nx rats. Although all prostaglandin levels except TXA2 decreased after administration of nicotine, they reached baseline levels only after COX-2 inhibition. (Fig. 5). Increased levels of TXA2 in the kidney are considered to be detrimental, and its inhibition has been shown to be beneficial in preventing the progression of kidney disease (7, 26, 36, 56), whereas vasodilatory prostaglandins such as PGI2 are considered beneficial and ameliorate renal injury. The novel finding in our study that the persistent elevation of cortical TXA2 in rats with CKD and that received nicotine, whereas all other prostaglandins are reduced, suggests that an imbalance among beneficial (i.e., PGI2) and harmful prostaglandins (TXA2) is a potential mechanism by which nicotine accelerates the progression of CKD. In support of this hypothesis, our study also demonstrated that COX-2 inhibition did not improve the severity of injury in rats with CKD on tap water, as in these rats all prostaglandins are increased and similarly inhibited by NS-398. In contrast to our findings in the renal cortex, medullary prostaglandins were not significantly increased by CKD or affected by nicotine (Fig. 6).
Previous studies have shown that increased oxidative stress plays an important role as a mediator of the effects of nicotine in CKD (27). In our study, we measured the urinary isoprostanes and nitrotyrosine expression to evaluate oxidative stress. We determined that although nicotine worsened oxidative stress in rats with CKD, NS-398 did not affect the urinary excretion of isoprostanes, supporting a previous report (37) showing that isoprostanes are produced by free radical mechanism independently from COX enzymes, and NS-398 did not modify the expression of nitrotyrosine. This data suggests that COX-2 is not an important source of oxidative stress in these rats and that the beneficial effect of COX-2 inhibition is independent from oxidative stress (Figs. 7 and 8).
In conclusion, the findings in this study unveil a novel mechanism by which nicotine accelerates the progression of CKD. Further studies will be needed to explore the benefits of targeting COX-2 in smokers and to alter the prostaglandins milieu to a more favorable balance to reduce the progression of CKD in smokers.
GRANTS
This work was supported by National Institutes of Health Grants RO1-ES-014948, P30-CA-008748, and P30-GM-103329.
DISCLOSURES
E.A.J. is cofounder, shareholder, and Chief Medical Officer of Goldilocks Therapeutics, Inc.
AUTHOR CONTRIBUTIONS
E.A.J. conceived and designed research; G.R., P.C., M.Y.G., W.F., and P.H. performed experiments; G.R., H.F., W.F., P.H., and E.A.J. analyzed data; S.R. and E.A.J. interpreted results of experiments; S.R., P.C., and E.A.J. prepared figures; S.R. drafted manuscript; E.A.J. edited and revised manuscript; E.A.J. approved final version of manuscript.
REFERENCES
- 1.Adams ML, Grandpre J, Katz DL, Shenson D. The impact of key modifiable risk factors on leading chronic conditions. Prev Med 120: 113–118, 2019. doi: 10.1016/j.ypmed.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89: 73–120, 2009. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baines RJ, Brunskill NJ. The molecular interactions between filtered proteins and proximal tubular cells in proteinuria. Nephron, Exp Nephrol 110: e67–e71, 2008. doi: 10.1159/000161982. [DOI] [PubMed] [Google Scholar]
- 4.Birn H, Willnow TE, Nielsen R, Norden AG, Bönsch C, Moestrup SK, Nexø E, Christensen EI. Megalin is essential for renal proximal tubule reabsorption and accumulation of transcobalamin-B12. Am J Physiol Renal Physiol 282: F408–F416, 2002. doi: 10.1152/ajprenal.00206.2000. [DOI] [PubMed] [Google Scholar]
- 5.Boddu R, Fan C, Rangarajan S, Sunil B, Bolisetty S, Curtis LM. Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am J Physiol Renal Physiol 313: F740–F755, 2017. doi: 10.1152/ajprenal.00049.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brose SA, Thuen BT, Golovko MY. LC/MS/MS method for analysis of E2 series prostaglandins and isoprostanes. J Lipid Res 52: 850–859, 2011. doi: 10.1194/jlr.D013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng H, Fan X, Guan Y, Moeckel GW, Zent R, Harris RC. Distinct roles for basal and induced COX-2 in podocyte injury. J Am Soc Nephrol 20: 1953–1962, 2009. doi: 10.1681/ASN.2009010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol 3: 258–267, 2002. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- 9.de Zeeuw D, Ramjit D, Zhang Z, Ribeiro AB, Kurokawa K, Lash JP, Chan J, Remuzzi G, Brenner BM, Shahinfar S. Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney Int 69: 1675–1682, 2006. doi: 10.1038/sj.ki.5000326. [DOI] [PubMed] [Google Scholar]
- 10.Dunn MJ. Prostaglandin I2 and the kidney. Arch Mal Coeur Vaiss 82: 27–31, 1989. [PubMed] [Google Scholar]
- 11.Dunn MJ, Hood VL. Prostaglandins and the kidney. Am J Physiol 233: 169–184, 1977. doi: 10.1152/ajprenal.1977.233.3.F169. [DOI] [PubMed] [Google Scholar]
- 12.Grando SA, Horton RM, Pereira EF, Diethelm-Okita BM, George PM, Albuquerque EX, Conti-Fine BM. A nicotinic acetylcholine receptor regulating cell adhesion and motility is expressed in human keratinocytes. J Invest Dermatol 105: 774–781, 1995. doi: 10.1111/1523-1747.ep12325606. [DOI] [PubMed] [Google Scholar]
- 13.Harris RC, Zhang MZ. Cyclooxygenase metabolites in the kidney. Compr Physiol 1: 1729–1758, 2011. doi: 10.1002/cphy.c100077. [DOI] [PubMed] [Google Scholar]
- 14.Hatzinikolaou DG, Lagesson V, Stavridou AJ, Pouli AE, Lagesson-Andrasko L, Stavrides JC. Analysis of the gas phase of cigarette smoke by gas chromatography coupled with UV-diode array detection. Anal Chem 78: 4509–4516, 2006. doi: 10.1021/ac052004y. [DOI] [PubMed] [Google Scholar]
- 15.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med 7: 833–839, 2001. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 16.Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest 110: 527–536, 2002. doi: 10.1172/JCI0214676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua P, Feng W, Ji S, Raij L, Jaimes EA. Nicotine worsens the severity of nephropathy in diabetic mice: implications for the progression of kidney disease in smokers. Am J Physiol Renal Physiol 299: F732–F739, 2010. doi: 10.1152/ajprenal.00293.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iseki K, Iseki C, Ikemiya Y, Fukiyama K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int 49: 800–805, 1996. doi: 10.1038/ki.1996.111. [DOI] [PubMed] [Google Scholar]
- 19.Jaimes EA, Tian RX, Joshi MS, Raij L. Nicotine augments glomerular injury in a rat model of acute nephritis. Am J Nephrol 29: 319–326, 2009. doi: 10.1159/000163593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaimes EA, Tian RX, Pearse D, Raij L. Up-regulation of glomerular COX-2 by angiotensin II: role of reactive oxygen species. Kidney Int 68: 2143–2153, 2005. doi: 10.1111/j.1523-1755.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 21.Jaimes EA, Tian RX, Raij L. Nicotine: the link between cigarette smoking and the progression of renal injury? Am J Physiol Heart Circ Physiol 292: H76–H82, 2007. doi: 10.1152/ajpheart.00693.2006. [DOI] [PubMed] [Google Scholar]
- 22.Jaimes EA, Zhou MS, Pearse DD, Puzis L, Raij L. Upregulation of cortical COX-2 in salt-sensitive hypertension: role of angiotensin II and reactive oxygen species. Am J Physiol Renal Physiol 294: F385–F392, 2008. doi: 10.1152/ajprenal.00302.2007. [DOI] [PubMed] [Google Scholar]
- 23.Jain G, Jaimes EA. Nicotine signaling and progression of chronic kidney disease in smokers. Biochem Pharmacol 86: 1215–1223, 2013. doi: 10.1016/j.bcp.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med 370: 60–68, 2014. doi: 10.1056/NEJMra1308383. [DOI] [PubMed] [Google Scholar]
- 25.Kogel U, Titz B, Schlage WK, Nury C, Martin F, Oviedo A, Lebrun S, Elamin A, Guedj E, Trivedi K, Ivanov NV, Vanscheeuwijck P, Peitsch MC, Hoeng J. Evaluation of the Tobacco Heating System 2.2. Part 7: systems toxicological assessment of a mentholated version revealed reduced cellular and molecular exposure effects compared with mentholated and non-mentholated cigarette smoke. Regul Toxicol Pharmacol 81, Suppl 2: S123–S138, 2016. doi: 10.1016/j.yrtph.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Kontessis PS, Jones SL, Barrow SE, Stratton PD, Alessandrini P, De Cosmo S, Ritter JM, Viberti GC. Effect of selective inhibition of thromboxane synthesis on renal function in diabetic nephropathy. J Lab Clin Med 121: 415–423, 1993. [PubMed] [Google Scholar]
- 27.Lan X, Lederman R, Eng JM, Shoshtari SS, Saleem MA, Malhotra A, Singhal PC. Nicotine induces podocyte apoptosis through increasing oxidative stress. PLoS One 11: e0167071, 2016. doi: 10.1371/journal.pone.0167071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lan X, Wen H, Aslam R, Shoshtari SSM, Mishra A, Kumar V, Wang H, Wu G, Luo H, Malhotra A, Singhal PC. Nicotine enhances mesangial cell proliferation and fibronectin production in high glucose milieu via activation of Wnt/β-catenin pathway. Biosci Rep 38: BSR20180100, 2018. doi: 10.1042/BSR20180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee WC, Lee YT, Li LC, Ng HY, Kuo WH, Lin PT, Liao YC, Chiou TT, Lee CT. The number of comorbidities predicts renal outcomes in patients with stage 3(-)5 chronic kidney disease. J Clin Med 7: 493, 2018. doi: 10.3390/jcm7120493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D, Wen Y, Tang TT, Lv LL, Tang RN, Liu H, Ma KL, Crowley SD, Liu BC. Megalin/cubulin-lysosome-mediated albumin reabsorption is involved in the tubular cell activation of NLRP3 inflammasome and tubulointerstitial inflammation. J Biol Chem 290: 18018–18028, 2015. doi: 10.1074/jbc.M115.662064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 33.Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther 287: 435–439, 1998. [PubMed] [Google Scholar]
- 34.Maus AD, Pereira EF, Karachunski PI, Horton RM, Navaneetham D, Macklin K, Cortes WS, Albuquerque EX, Conti-Fine BM. Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol Pharmacol 54: 779–788, 1998. doi: 10.1124/mol.54.5.779. [DOI] [PubMed] [Google Scholar]
- 35.Meran S, Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol 92: 158–167, 2011. doi: 10.1111/j.1365-2613.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morio H, Hirai A, Terano T, Tamura Y, Yoshida S. Effect of the infusion of OKY-046, a thromboxane A2 synthase inhibitor, on urinary metabolites of prostacyclin and thromboxane A2 in healthy human subjects. Thromb Haemost 69: 276–281, 1993. doi: 10.1055/s-0038-1651595. [DOI] [PubMed] [Google Scholar]
- 37.Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ II. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci USA 89: 10721–10725, 1992. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motoyoshi Y, Matsusaka T, Saito A, Pastan I, Willnow TE, Mizutani S, Ichikawa I. Megalin contributes to the early injury of proximal tubule cells during nonselective proteinuria. Kidney Int 74: 1262–1269, 2008. doi: 10.1038/ki.2008.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen R, Christensen EI, Birn H. Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int 89: 58–67, 2016. doi: 10.1016/j.kint.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Obert DM, Hua P, Pilkerton ME, Feng W, Jaimes EA. Environmental tobacco smoke furthers progression of diabetic nephropathy. Am J Med Sci 341: 126–130, 2011. doi: 10.1097/MAJ.0b013e3181f6e3bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orth SR. Smoking--a renal risk factor. Nephron 86: 12–26, 2000. doi: 10.1159/000045708. [DOI] [PubMed] [Google Scholar]
- 42.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 123: 754–762, 1995. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 43.Raij L, Chiou XC, Owens R, Wrigley B. Therapeutic implications of hypertension-induced glomerular injury. Comparison of enalapril and a combination of hydralazine, reserpine, and hydrochlorothiazide in an experimental model. Am J Med 79: 37–41, 1985. doi: 10.1016/0002-9343(85)90078-6. [DOI] [PubMed] [Google Scholar]
- 44.Rezonzew G, Chumley P, Feng W, Hua P, Siegal GP, Jaimes EA. Nicotine exposure and the progression of chronic kidney disease: role of the α7-nicotinic acetylcholine receptor. Am J Physiol Renal Physiol 303: F304–F312, 2012. doi: 10.1152/ajprenal.00661.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31: 986–1000, 2011. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roman J, Ritzenthaler JD, Gil-Acosta A, Rivera HN, Roser-Page S. Nicotine and fibronectin expression in lung fibroblasts: implications for tobacco-related lung tissue remodeling. FASEB J 18: 1436–1438, 2004. doi: 10.1096/fj.03-0826fje. [DOI] [PubMed] [Google Scholar]
- 47.Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care 25: 859–864, 2002. doi: 10.2337/diacare.25.5.859. [DOI] [PubMed] [Google Scholar]
- 48.Ruggenenti P, Perna A, Mosconi L, Matalone M, Pisoni R, Gaspari F, Remuzzi G. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int Suppl 63: S54–S57, 1997. [PubMed] [Google Scholar]
- 49.Storm T, Tranebjærg L, Frykholm C, Birn H, Verroust PJ, Nevéus T, Sundelin B, Hertz JM, Holmström G, Ericson K, Christensen EI, Nielsen R. Renal phenotypic investigations of megalin-deficient patients: novel insights into tubular proteinuria and albumin filtration. Nephrol Dial Transplant 28: 585–591, 2013. doi: 10.1093/ndt/gfs462. [DOI] [PubMed] [Google Scholar]
- 50.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest 108: 15–23, 2001. doi: 10.1172/JCI200113416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Vliet A, Baelde HJ, Vleming LJ, de Heer E, Bruijn JA. Distribution of fibronectin isoforms in human renal disease. J Pathol 193: 256–262, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 52.Wagenknecht LE, Cutter GR, Haley NJ, Sidney S, Manolio TA, Hughes GH, Jacobs DR. Racial differences in serum cotinine levels among smokers in the Coronary Artery Risk Development in (Young) Adults study. Am J Public Health 80: 1053–1056, 1990. doi: 10.2105/AJPH.80.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wessler I, Kirkpatrick CJ, Racké K. The cholinergic ‘pitfall’: acetylcholine, a universal cell molecule in biological systems, including humans. Clin Exp Pharmacol Physiol 26: 198–205, 1999. doi: 10.1046/j.1440-1681.1999.03016.x. [DOI] [PubMed] [Google Scholar]
- 54.Ying WZ, Li X, Rangarajan S, Feng W, Curtis LM, Sanders PW. Immunoglobulin light chains generate proinflammatory and profibrotic kidney injury. J Clin Invest 129: 2792–2806, 2019. doi: 10.1172/JCI125517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou MS, Chadipiralla K, Mendez AJ, Jaimes EA, Silverstein RL, Webster K, Raij L. Nicotine potentiates proatherogenic effects of oxLDL by stimulating and upregulating macrophage CD36 signaling. Am J Physiol Heart Circ Physiol 305: H563–H574, 2013. doi: 10.1152/ajpheart.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zoja C, Perico N, Corna D, Benigni A, Gabanelli M, Morigi M, Bertani T, Remuzzi G. Thromboxane synthesis inhibition increases renal prostacyclin and prevents renal disease progression in rats with remnant kidney. J Am Soc Nephrol 1: 799–807, 1990. [DOI] [PubMed] [Google Scholar]