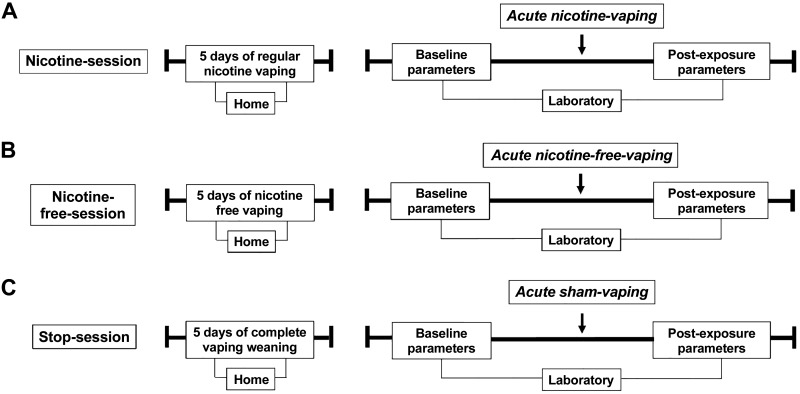
Fig. 1.
Typical course of the three experimental periods. The periods consisted of regular nicotine e-cigarette vaping for 5 days before the experimental session (nicotine-session) (A); nicotine-free-vaping for 5 days before the experimental session (nicotine-free-session) (B); and complete cessation of e-cigarette vaping for 5 days before the experimental session (stop-session) (C). Biological/clinical cardiorespiratory parameters (serum/urine pneumoproteins, continuous hemodynamic parameters, transcutaneous gas tensions, and skin microcirculatory blood flow) were assessed at the beginning of each session and after acute vaping exposure: 10 nicotine puffs (acute nicotine-vaping in the nicotine-session) (A); 10 nicotine-free puffs (acute nicotine-free-vaping in the nicotine-free-session) (B); and 10 sham puffs (acute sham-vaping in the stop-session) (C).