Abstract
Background
Clinical trials show high efficacy of sofosbuvir/velpatasvir (SOF/VEL), but there are limited data from “real-world” settings. We aimed to evaluate SOF/VEL effectiveness for all hepatitis C virus (HCV) genotypes (GTs) in British Columbia (BC), Canada.
Methods
We used the BC Hepatitis Testers Cohort, which includes all HCV cases in the province (1990–2015) linked to administrative databases, including prescriptions to end of 2018. We measured sustained virologic response (SVR; negative RNA ≥10 weeks after treatment end) and identified characteristics associated with non-SVR. Conservatively, we excluded individuals with no assessment for SVR if their last RNA test after treatment initiation was negative (but included if positive).
Results
Of 2821 eligible participants, most were infected with GT1 (1076, 38.1%) or GT3 (1072, 38.0%), and a minority (278, 9.9%) were treated with RBV. SVR was 94.6% (2670/2821) overall and 94.5% (1017/1076) for GT1, 96.4% (512/531) for GT2, and 93.7% (1004/1072) for GT3. When disaggregated by GT, treatment regimen, and cirrhosis/treatment experience, SVR was lowest (30/40, 75.0%) among treatment-experienced GT3 individuals treated with RBV. Characteristics associated with non-SVR in multivariable analysis included younger age, RBV addition, and being a person with HIV (PWH) or who injects/injected drugs (PWID). When treatment regimen (±RBV) was removed from multivariable model, treatment experience was associated with non-SVR for GT3. Of 151 non-SVR individuals, 56.3% were nonvirological failures (treatment incomplete/no assessment for SVR) and 43.7% were virological failures (nonresponse/relapse). A disproportionately high percentage of non-SVR among PWID was due to nonvirological failure.
Conclusions
SOF/VEL was highly effective in this “real-world” population-based cohort. Additional support is required for PWID/PWH to reach SVR.
Keywords: hepatitis C, treatment effectiveness, observational study, administrative data, sofosbuvir, velpatasvir, ribavirin
Hepatitis C virus (HCV) and its associated morbidity and mortality are a significant public health issue in Canada and globally [1, 2]. In the absence of effective treatment, about 75% of individuals infected with HCV advance to chronic infection, and 10%–15% of chronically infected individuals develop liver cirrhosis within 20 years [3]. People with cirrhosis are at higher risk of end-stage liver disease and death.
Second-generation oral direct-acting antivirals (DAAs) are short course (8–24 weeks), have fewer adverse effects, and can cure >95% of people with HCV. This high cure rate and ease of use led the World Health Organization to issue the first Global Health Sector Strategy on Viral Hepatitis, with the aim of eliminating HCV as a major public health issue by 2030 [4]. Sofosbuvir/velpatasvir (SOF/VEL) is the first of these DAAs to be pan-genotypic and require a single pill taken once daily. SOF/VEL clinical trials published in 2015 demonstrated sustained virologic response (SVR) rates exceeding 97% for all genotypes (GTs) except for GT3 (94.7%) [5–10]. In 2016, SOF/VEL was approved by regulatory agencies in the United States (Food and Drug Administration), Canada (Health Canada), and the European Union (European Commission) [11, 12].
Although clinical trials show high efficacy of SOF/VEL, actual SVR in real-world clinical settings could be lower due to differences in patient populations, resources, and adherence to best practices [13, 14]. However, few large published studies have evaluated SOF/VEL in real-world settings using conservative intention-to-treat (ITT) approaches [15], and, to the best of our knowledge, none have used a population-based data source (ie, included all treated individuals within a jurisdiction). Conservative analytic approaches do not exclude individuals lost to follow-up and are critical for understanding real-world factors contributing to lack of SVR [9, 15]. Conservative, real-world studies are particularly important to evaluate effectiveness in populations that often experience worse treatment outcomes for biological and/or social reasons, people with cirrhosis, decompensated disease, GT3 infection and/or prior history of HCV treatment, as well as people who inject drugs. Further, there are limited data available from real-world settings on the effectiveness of SOF/VEL against various genotypes, and it is unclear from real-world experience whether the addition of ribavirin (RBV) to SOF/VEL improves SVR [15–17]. Finally, lack of population-based analyses limits generalizability of study results to all real-world practice within a jurisdiction. Therefore, additional studies are needed to evaluate SOF/VEL effectiveness outside of controlled clinical trial settings to inform decisions at the clinician, programming, and policy levels.
In this study, we use a large population-based cohort and a conservative analytic approach to evaluate the effectiveness SOF/VEL±RBV in real-world clinical practice in British Columbia (BC).
METHODS
Setting
BC is Canada’s third largest province, with a population of almost 5 million in 2018. The province’s rate of 48.5 new HCV diagnoses per 100 000 people is 55% higher than the national rate and the second highest in the country [18]. SOF/VEL became available in BC on July 14, 2016. Public coverage of SOF/VEL for certain populations started on April 2017 and was expanded to all HCV-positive individuals on April 2018 [19–21]. SOF/VEL±RBV is prescribed for treatment of all genotypes for 12 weeks. Treatment decisions are made at the clinician’s discretion, with decisions guided by Canadian (CASL), American (AASLD), and European (EASL) guidelines [22–24]. In general, the addition of RBV to SOF/VEL is considered standard of care for decompensated patients, and is also accessible for those with GT3 cirrhosis.
Data Sources
We used data from the BC Hepatitis Testers Cohort (BC-HTC). Details related to cohort creation and epidemiological characteristics have been reported previously [25]. The BC-HTC inclusion criteria and data sources are also summarized in Supplementary Table 1. In brief, the cohort includes all individuals tested for HCV or HIV or reported as a case of hepatitis B virus (HBV), HCV, HIV, or active tuberculosis in BC between 1990 and 2015. These data are integrated with medical visits, hospitalizations, cancers, prescription drugs, deaths, and BC Centre for Disease Control Public Health Laboratory (BCCDC-PHL) testing data. The data set used for this analysis included prescription and death data updated until the end of 2018 and BCCDC-PHL HCV laboratory tests updated to April 9, 2019.
All residents in BC are registered in the publicly funded insurance plan, which acts as a single-payer system and covers services provided by fee-for-service practitioners. HCV screening and RNA testing for the entire province are performed at BCCDC-PHL, except for <5% of screening tests performed at a regional laboratory that sends positive tests to BCCDC-PHL for confirmation and HCV RNA testing. All dispensed prescriptions in the province, including HCV treatments, are recorded in a central system called PharmaNet.
Study Population and Treatment
In this analysis, we included HCV-positive individuals who were in the cohort as of the end of 2015. HCV-positive individuals were defined as those who had tested positive for HCV antibodies, had undergone HCV RNA or genotype testing, or were reported as a case of HCV to public health. Our data sets included data on SOF/VEL treatment through December 31, 2018, and HCV RNA testing data through April 9, 2019. To allow for adequate follow-up time (at least 12 weeks to assess treatment completion and 12 weeks to assess SVR), we excluded individuals initiating SOF/VEL after October 9, 2018.
Modified Intention-to-Treat Analysis and SVR Definition
Achievement of SVR was defined as any record of a negative (below the lower limit of detection) HCV RNA ≥10 weeks after treatment end. As in other studies, a 10-week time period, instead of 12 weeks, was chosen to account for variability in testing in clinical practice [26]. Of individuals with an RNA test at week 10 or later in our data, only 1.9% (52/2750) were tested at weeks 10–11 and not ≥12 weeks.
Our modified ITT approach and non-SVR definition were conservative, similar to others [9, 15]. We excluded those with (1) no RNA test after treatment initiation or (2) a negative RNA test on their last test (either while on treatment or after treatment end) but no assessment for SVR (ie, no RNA test ≥10 weeks after treatment end). Participants included in the analysis were categorized as not achieving SVR (non-SVR) if they had (1) any detectable HCV RNA after the end of treatment, (2) a positive RNA test during treatment and no viral load test after the end of treatment, or (3) a detectable HCV RNA on their last HCV viral load test (either while on treatment or within 10 weeks of the treatment end). Therefore, individuals with no assessment for SVR were excluded if their last RNA test was negative, but included if the RNA test was positive.
Plasma HCV RNA levels were determined using the Abbott RealTime HCV assay (Abbott Molecular Inc., Mississauga, ON, Canada), with a lower limit of detection for HCV RNA of 12 IU/mL.
Non-SVR Categories
We assessed the most likely reason for non-SVR using 5 hierarchical, mutually exclusive categories. A completed treatment course was defined as ≥12 weeks of treatment, and SVR assessment was defined as a positive/negative RNA test ≥10 weeks after treatment end. The categories were defined as follows:
- A) Nonvirological failures (criteria: treatment incomplete and/or no assessment for SVR, and received a positive RNA test after treatment initiation).
- Incomplete treatment (for reasons other than death): Individuals who did not complete treatment and did not die within 12 weeks of treatment initiation.
- Death prevented treatment completion/SVR assessment: Individuals who did not receive SVR assessment and died either during treatment or within 14 weeks after treatment end.
- Lost to follow-up (LTFU; for reasons other than death): Individuals who completed treatment, did not receive SVR assessment, and did not die either during treatment or within 14 weeks after treatment end.
- B) Virological failures (criteria: completed treatment and received a positive RNA test ≥10 weeks after treatment end).
- Relapse: Individuals who received a negative RNA test either during or after treatment (but before 10 weeks after treatment end).
- Nonresponse: Individuals who did not receive a negative RNA test either during or after treatment.
Assessment of Covariates
Demographic characteristics included sex, age, birth cohort, and social and material deprivation quintiles. Assessment of diabetes, history of injecting drugs, major mental illness, cirrhosis, decompensated cirrhosis, and problematic alcohol use were based on algorithms derived from medical visits, hospitalization, or prescription dispensation data using fee-for-service, procedure, and/or diagnostic codes (Supplementary Table 2).
Analysis
We computed SVR overall and by GT and compared the proportion achieving SVR across a range of participant characteristics. We performed multivariable logistic regression analyses to identify predictors of non-SVR.
All analyses were conducted in SAS/STAT, version 9.4, and all tests were 2-sided at a significance level of .05. This study was approved by the University of British Columbia Research Ethics Board (H14-01649).
RESULTS
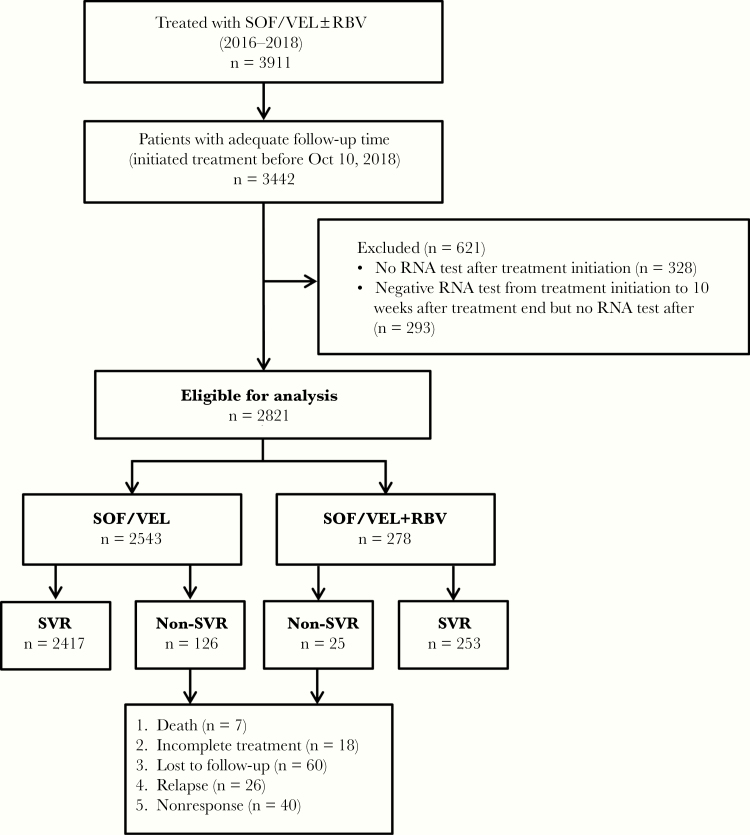
Overall, 3911 unique HCV-positive individuals initiated SOF/VEL±RBV treatment on or before December 31, 2018 (Figure 1). Of 3442 with adequate follow-up time (initiated treatment on or before October 9, 2018), a total of 621 (18.0%) were excluded. Differences between included and excluded individuals are shown in Supplementary Table 3. Of note, excluded individuals (particularly those with a negative RNA test) were more likely to be PWID.
Figure 1.
Study flowchart, BC Hepatitis Testers Cohort, 2016–2018. Abbreviations: BC, British Columbia; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virologic response; VEL, velpatasvir.
A total of 2821 eligible participants were included in the study, and their characteristics are shown in Table 1. Most were infected with either GT1 (38.1%) or GT3 (38.0%), followed by GT2 (18.8%) and GT4-6 (5.1%). The majority of participants were male (62.6%), white (90.6%), aged 50+ years (76.3%), and born between 1945 and 1964 (65.3%).
Table 1.
Characteristics of Cohort Participants Treated With SOF/VEL±RBV by Genotype, BC Hepatitis Testers Cohort, 2016–2018
| Overall, No. (%) | GT1, No. (%) | GT2, No. (%) | GT3, No. (%) | |
|---|---|---|---|---|
| Total, No. | 2821 | 1076 | 531 | 1072 |
| Birth cohort | ||||
| <1945 | 104 (3.7) | 26 (2.4) | 36 (6.8) | 24 (2.3) |
| 1945–1964 | 1843 (65.3) | 734 (68.2) | 408 (76.9) | 621 (58) |
| 1965–1974 | 525 (18.6) | 200 (18.6) | 64 (12) | 237 (22.1) |
| ≥1975 | 340 (12.4) | 116 (10.8) | 23 (4.4) | 190 (17.7) |
| Age, median (IQR), y | 58 (50–63) | 58 (51–63) | 61 (56–66) | 55 (47–61) |
| Age | ||||
| <50 | 667 (23.7) | 234 (21.7) | 60 (11.3) | 337 (31.4) |
| 50–60 | 1086 (38.5) | 410 (39) | 187 (35.2) | 440 (41) |
| >60 | 1068 (37.8) | 423 (39.3) | 284 (53.5) | 295 (27.5) |
| Sex | ||||
| Female | 1055 (37.4) | 360 (34.3) | 225 (42.4) | 406 (37.9) |
| Male | 1766 (62.6) | 707 (65.7) | 306 (57.6) | 666 (62.1) |
| Ethnicity | ||||
| White | 2555 (90.6) | 1030 (95.7) | 493 (92.8) | 961 (89.7) |
| Others | 266 (9.5) | 46 (4.3) | 38 (7.2) | 111 (10.4) |
| Treatment regimen | ||||
| SOF/VEL | 2543 (90.2) | 976 (90.7) | 500 (94.2) | 934 (87.1) |
| SOF/VEL+RBV | 278 (9.9) | 100 (9.3) | 31 (5.8) | 138 (12.9) |
| HCV treatment experience | 310 (11.0) | 108 (10.1) | 51 (9.6) | 137 (12.8) |
| Cirrhosis | 105 (3.7) | 26 (2.4) | 21 (4.0) | 53 (5.0) |
| Decompensated cirrhosis | 66 (2.3) | 18 (1.7) | 12 (2.3) | 33 (3.1) |
| OST | ||||
| Recent | 630 (22.3) | 226 (21) | 50 (11.1) | 322 (30.1) |
| Past | 168 (5.9) | 60 (5.6) | 25 (4.7) | 78 (7.3) |
| None | 2023 (71.7) | 790 (73.4) | 447 (84.1) | 672 (62.7) |
| HBV | 187 (6.7) | 50 (5.5) | 36 (6.8) | 76 (7.1) |
| HIV | 248 (8.8) | 80 (8.3) | 21 (4) | 123 (11.4) |
| Diabetes | 354 (12.6) | 128 (11.9) | 71 (13.4) | 128 (11.9) |
| History of injecting drugs | 1021 (36.1) | 370 (35.2) | 131 (24.6) | 478 (44.6) |
| Problematic alcohol use | 833 (29.6) | 333 (30.9) | 134 (25.2) | 343 (32) |
| Mental illness | 987 (35) | 376 (34.9) | 150 (29.9) | 423 (39.5) |
| Statin use | 357 (12.6) | 140 (13) | 80 (16.8) | 102 (9.5) |
| Material deprivation | ||||
| Unknown | 34 (1.2) | 15 (1.4) | 5 (1) | 13 (1.2) |
| Q1 (least) | 384 (13.6) | 146 (13.6) | 78 (14.6) | 140 (13) |
| Q2 | 434 (15.4) | 176 (16.4) | 90 (18.7) | 138 (12.9) |
| Q3 | 530 (19.1) | 198 (18.4) | 103 (19.4) | 204 (19) |
| Q4 | 686 (24.4) | 272 (25.2) | 126 (23.7) | 257 (24) |
| Q5 (most) | 744 (26.4) | 260 (25) | 120 (22.6) | 320 (29.9) |
| Social deprivation | ||||
| Unknown | 34 (1.2) | 15 (1.4) | 5 (1) | 13 (1.2) |
| Q1 (least) | 270 (9.9) | 83 (7.7) | 45 (8.5) | 126 (11.7) |
| Q2 | 335 (11.9) | 118 (11) | 78 (14.7) | 112 (10.5) |
| Q3 | 458 (16.3) | 186 (17.3) | 110 (20.8) | 140 (13) |
| Q4 | 628 (22.3) | 248 (23) | 120 (22.6) | 231 (21.6) |
| Q5 (most) | 1087 (38.6) | 426 (39.6) | 173 (32.6) | 450 (42) |
| Elixhauser index | ||||
| 0 | 1223 (43.4) | 464 (43.1) | 243 (45.8) | 435 (40.5) |
| ≥1 | 1598 (56.7) | 612 (56.9) | 288 (54.3) | 637 (59.4) |
Abbreviations: BC, British Columbia; GT, genotype; HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; OST, opioid substitution therapy; OST, opioid substitution therapy; Q, quintile; RBV, ribavirin; SOF, sofosbuvir; VEL, velpatasvir; y, year.
HCV Treatment
Overall, 98.0% of participants completed a treatment course ≥12 weeks, and 278 (9.9%) were treated with RBV. Treatment completion was lower for non-SVR vs SVR (86.1% vs 98.6%; P < .0001) and SOF/VEL+RBV vs SOF/VEL alone (94.6% vs 98.3%; P < .0001). Of non-SVR individuals who lacked SVR assessment due to death/LTFU, 82.5% had completed treatment. Differences between individuals treated with vs without RBV are shown in Supplementary Table 4.
SVR Rates
SVR was 94.6% (2670/2821) overall and 94.5% (1017/1076) for GT1, 96.4% (512/531) for GT2, and 93.7% for GT3 (1004/1072) (Table 2). For GT1, SVR was lowest for individuals with HIV co-infection (84.3%, 75/89), recent opioid substitution therapy (87.2%, 197/226), cirrhosis (88.5%, 23/26), decompensated cirrhosis (88.9%, 16/18), and history of injecting drugs (89.2%, 338/379). For GT3, SVR was lowest for those who were born pre-1945 (79.2%, 19/24), treated with RBV (87.0%, 120/138), and HCV treatment-experienced (89.8%, 123/137).
Table 2.
SVR Rates for SOF/VEL±RBV by Genotype and Participant Characteristics, BC Hepatitis Testers Cohort, 2016–2018
| GT1 | GT2 | GT3 | |
|---|---|---|---|
| % (n/N) | % (n/N) | % (n/N) | |
| Overall | 94.5 (1017/1076) | 96.4 (512/531) | 93.7 (1004/1072) |
| Birth cohort | |||
| <1945 | 100 (26/26) | 94.4 (34/36) | 79.2 (19/24) |
| 1945–1964 | 95.9 (704/734) | 97.5 (398/408) | 94.7 (588/621) |
| 1965–1974 | 91.5 (183/200) | 92.2 (59/64) | 93.7 (222/237) |
| ≥1975 | 89.7 (104/116) | 91.3 (21/23) | 92.1 (175/190) |
| Age, y | |||
| <50 | 89.7 (210/234) | 88.3 (53/60) | 92.0 (310/337) |
| 50–60 | 96.2 (403/419) | 99.5 (186/187) | 94.8 (417/440) |
| >60 | 95.5 (404/423) | 96.1 (273/284) | 93.9 (277/295) |
| Sex | |||
| Female | 95.9 (354/369) | 96 (216/225) | 95.1 (386/406) |
| Male | 93.8 (663/707) | 96.7 (296/306) | 92.8 (618/666) |
| Ethnicity | |||
| White | 94.4 (972/1030) | 96.6 (476/493) | 93.4 (898/961) |
| Others | 97.8 (45/46) | 94.7 (36/38) | 95.5 (106/111) |
| Treatment regimen | |||
| SOF/VEL | 94.6 (923/976) | 96.2 (481/500) | 94.7 (884/934) |
| SOF/VEL+RBV | 94.0 (94/100) | 100 (31/31) | 87.0 (120/138) |
| HCV treatment experience | |||
| No | 94.6 (916/968) | 96.3 (462/480) | 94.2 (881/935) |
| Yes | 93.5 (101/108) | 98 (50/51) | 89.8 (123/137) |
| Cirrhosis | |||
| No | 94.7 (994/1050) | 96.3 (491/510) | 93.6 (954/1019) |
| Yes | 88.5 (23/26) | 100 (21/21) | 94.3 (50/53) |
| Decompensated cirrhosis | |||
| No | 94.6 (1001/1058) | 96.3 (500/519) | 93.5 (971/1039) |
| Yes | 88.9 (16/18) | 100.0 (12/12) | 100.0 (33/33) |
| OST | |||
| Recent | 87.2 (197/226) | 91.5 (54/59) | 93.8 (302/322) |
| Past | 95 (57/60) | 96 (24/25) | 97.4 (76/78) |
| None | 96.6 (763/790) | 97.1 (434/447) | 93.2 (626/672) |
| HBV | |||
| No | 94.7 (963/1017) | 96.2 (476/495) | 93.7 (933/996) |
| Yes | 91.5 (54/59) | 100 (36/36) | 93.4 (71/76) |
| HIV | |||
| No | 95.4 (942/987) | 96.5 (492/510) | 93.8 (890/949) |
| Yes | 84.3 (75/89) | 95.2 (20/21) | 92.7 (114/123) |
| Diabetes | |||
| No | 94.3 (894/948) | 96.1 (442/460) | 94.0 (887/944) |
| Yes | 96.1 (123/128) | 98.6 (70/71) | 91.4 (117/128) |
| History of injecting drugs | |||
| No | 97.4 (679/697) | 96.8 (387/400) | 94.6 (562/594) |
| Yes | 89.2 (338/379) | 95.4 (125/131) | 92.5 (442/478) |
| Problematic alcohol use | |||
| No | 95.2 (707/743) | 96.5 (383/397) | 93.3 (680/729) |
| Yes | 93.1 (310/333) | 96.3 (129/134) | 94.5 (324/343) |
| Mental illness | |||
| No | 94.9 (664/700) | 96.5 (359/372) | 94.0 (610/649) |
| Yes | 93.9 (353/376) | 96.2 (153/159) | 93.1 (394/423) |
| Statin | |||
| No | 94.6 (885/936) | 96.2 (425/442) | 94.0 (912/970) |
| Yes | 94.3 (132/140) | 97.8 (87/89) | 90.2 (92/102) |
| Material deprivation | |||
| Unknown | 100 (15/15) | 60 (3/5) | 92.3 (12/13) |
| Q1 (least) | 96.6 (141/146) | 93.6 (73/78) | 94.3 (132/140) |
| Q2 | 96.6 (170/176) | 97 (96/99) | 91.3 (126/138) |
| Q3 | 93.9 (186/198) | 97.1 (100/103) | 95.6 (195/204) |
| Q4 | 91.9 (250/272) | 99.2 (125/126) | 93.8 (241/257) |
| Q5 (most) | 94.8 (255/269) | 95.8 (115/120) | 93.1 (298/320) |
| Social deprivation | |||
| Unknown | 100 (15/15) | 60 (3/5) | 92.3 (12/13) |
| Q1 (least) | 95.2 (79/83) | 93.3 (42/45) | 93.7 (118/126) |
| Q2 | 96.6 (114/118) | 96.2 (75/78) | 97.3 (109/112) |
| Q3 | 96.2 (179/186) | 96.4 (106/110) | 90.7 (127/140) |
| Q4 | 96 (238/248) | 100 (120/120) | 93.1 (215/231) |
| Q5 (most) | 92 (392/426) | 96 (166/173) | 94.0 (423/450) |
| Elixhauser index | |||
| 0 | 96.8 (449/464) | 96.3 (234/243) | 94.3 (410/435) |
| ≥1 | 92.8 (568/612) | 96.5 (278/288) | 93.2 (594/637) |
Bold formatting indicates percentage <90%.
Abbreviations: BC, British Columbia; GT, genotype; HBV, hepatitis B virus; HCV, hepatitis C virus; OST, opioid substitution therapy; Q, quintile; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virologic response; VEL, velpatasvir; y, year.
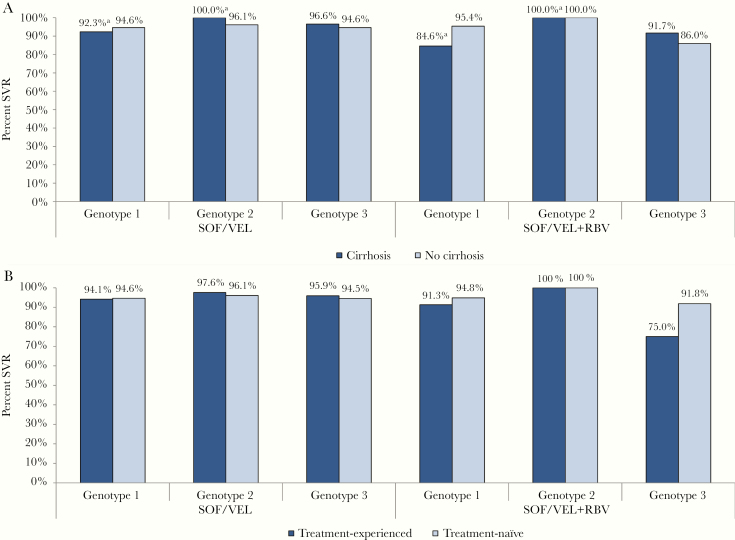
Figure 2A shows the percentage achieving SVR by treatment regimen, GT, and cirrhotic state. SVR rates were ≥90% for all populations except GT1 cirrhotic patients treated with RBV (84.6%, 11/13) and GT3 noncirrhotic patients treated with RBV (86.0%, 98/114). In contrast to the latter, GT3 noncirrhotic patients treated without RBV had an SVR rate of 94.6% (856/905).
Figure 2.
SVR rates by genotype, treatment regimen, and (A) cirrhotic state or (B) HCV treatment experience, BC Hepatitis Testers Cohort, 2016–2018. “Cirrhosis” includes those with compensated or decompensated cirrhosis. aDenominator <15. Abbreviations: BC, British Columbia; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virologic response; VEL, velpatasvir.
Figure 2B shows the percentage achieving SVR by treatment regimen, GT, and HCV treatment history. SVR rates were ≥90% for all populations except for treatment-experienced GT3 patients treated with RBV (75.0%, 30/40). In further analysis of the latter, SVR was 88.9% (16/18) for those previously treated with DAAs and 63.6% (14/22) for those treated with interferon-based regimens. In contrast, treatment-experienced GT3 patients treated without RBV had an SVR rate of 95.9% (93/97).
In sensitivity analyses, inclusion of the 293 excluded individuals with a negative RNA test but no SVR assessment increased the SVR rate (if assumed to have achieved SVR) from 94.6% to 95.2% overall and from 91.6% to 93.5% among PWID—who are disproportionately represented among this excluded population. Of note, 98.6% of the 293 excluded individuals had completed ≥12 weeks of treatment, and the median (interquartile range [IQR]) time from treatment initiation to negative RNA test was 15 (13–18) weeks.
Predictors of Non-SVR
Characteristics associated with non-SVR in multivariable models are shown in Table 3. In the overall model, age <50 years (vs >60 years; adjusted odds ratio [aOR], 1.58; 95% confidence interval [CI], 1.00–2.48), history of injecting drugs (aOR, 2.35; 95% CI, 1.51–3.66), HIV co-infection (aOR, 1.67; 95% CI, 1.02–2.75), and treatment with RBV (vs SOF/VEL alone; aOR, 1.93; 95% CI, 1.19–3.13) were associated with non-SVR. In GT1- and GT3-specific models, factors associated with non-SVR included history of injecting drugs (GT1, GT3), HIV co-infection (GT1), and treatment with RBV (GT3). GT, cirrhosis, and HCV treatment experience were not associated with non-SVR in any model.
Table 3.
Logistic Regression Analyses Assessing Characteristics Associated With Non-SVR Among Individuals Treated With SOF/VEL, BC Hepatitis Testers Cohort, 2016–2018
| Overall | GT1 | GT3 | |
|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Age (vs >60 y), y | |||
| <50 | 1.58 (1.00–2.48) | 1.23 (0.60–2.56) | 1.82 (0.90–3.68) |
| 50–60 | 0.64 (0.41–1.01) | 0.53 (0.26–1.12) | 0.88 (0.45–1.72) |
| Sex (male vs female) | 1.41 (0.98–2.02) | 1.79 (0.95–3.35) | 1.49 (0.86–2.59) |
| HCV treatment experience (yes vs no) | 1.36 (0.83–2.24) | 1.14 (0.46–2.8) | 1.73 (0.89–3.36) |
| Cirrhosis (yes vs no) | 1.21 (0.52–2.80) | 2.64 (0.66–10.59) | 0.66 (0.19–2.34) |
| History of injecting drugs (yes vs no) | 2.35 (1.51–3.66) | 3.82 (1.8–8.12) | 2.32 (1.24–4.36) |
| OST (vs none) | |||
| Recent | 1.08 (0.70–1.66) | 1.69 (0.85–3.35) | 0.52 (0.27–1.01) |
| Past | 1.36 (0.83–2.24) | 0.77 (0.21–2.77) | 0.23 (0.05–1.02) |
| Problematic alcohol use (yes vs no) | 0.74 (0.5–1.09) | 0.72 (0.39–1.34) | 0.61 (0.33–1.12) |
| HIV (yes vs no) | 1.67 (1.02–2.75) | 2.39 (1.13–5.04) | 1.06 (0.48–2.35) |
| HBV (yes vs no) | 0.74 (0.37–1.49) | 0.76 (0.26–2.22) | 1.16 (0.43–3.14) |
| Genotype (vs GT1) | |||
| GT2 | 0.78 (0.46–1.35) | NA | NA |
| GT3 | 1.00 (0.69–1.44) | NA | NA |
| GT4-6 | 0.64 (0.25–1.66) | NA | NA |
| SOF/VEL+RBV (vs SOF/VEL) | 1.93 (1.19–3.13) | 0.97 (0.38–2.53) | 3.10 (1.67–5.76) |
Bold formatting indicates P < .05. When treatment variable (SOF/VEL vs SOF/VEL+RBV) was removed from the model, “HCV treatment experience” was significant for the GT3 model (aOR, 2.03; 95% CI, 1.06–3.89). GT2 is not shown.
Abbreviations: aOR, adjusted odds ratio; BC, British Columbia; CI, confidence interval; GT, genotype; HBV, hepatitis B virus; HCV, hepatitis C virus; NA, not applicable; OST, opioid substitution therapy; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virologic response; VEL, velpatasvir.
In sensitivity analyses, model results were largely unchanged when the treatment regimen variable (SOF/VEL+RBV vs SOF/VEL alone) was removed from the models (data not shown), with 1 exception. After removal of treatment regimen from the GT3 model, treatment experience was associated with non-SVR (vs treatment-naïve; aOR, 2.03; 95% CI, 1.06–3.89). Further, an interaction term between treatment regimen and HCV treatment experience was significant in the full and GT3-specific models (data not shown). The interaction showed that SOF/VEL+RBV (vs SOF/VEL alone) was associated with non-SVR in treatment-experienced individuals (aOR, 11.5; 95% CI, 3.2–41.6; full model) but not treatment-naïve individuals (aOR, 1.9; 95% CI, 0.8–4.2; full model). In an additional sensitivity analysis, the model results were also largely unchanged when the 293 excluded individuals (negative RNA test post–treatment initiation but no SVR assessment) were included and assumed to have achieved SVR (data not shown).
Reasons for Non-SVR
A total of 151 individuals did not achieve SVR. Overall, 56.3% were nonvirological failures, and 43.7% were virological failures. The underlying reasons for non-SVR were as follows: 18 (11.9%) did not complete treatment (for reasons other than death), death prevented treatment completion and/or SVR assessment for 7 (4.6%) individuals, 60 (39.7%) completed treatment but lacked SVR assessment (LTFU), 26 (17.2%) relapsed, and 40 (26.5%) were nonresponders. A total of 86.1% of nonvirological failures completed a treatment course ≥12 weeks. The median (IQR) duration of treatment was 4 (4–8) weeks for those who did not complete treatment. Among all nonvirological failures, the median (IQR) time from treatment initiation to positive RNA test was 11 (5–16) weeks.
A comparison of SVR and non-SVR populations by select characteristics is shown in Supplementary Tables 5 and 6. Of note, people who inject/injected drugs (PWID) composed 35.0% of the SVR population but 57.0% of non-SVR cases (63.5% of nonvirological failures and 48.5% of virological failures; 62.8% of non-SVR among PWID was due to nonvirological failure).
DISCUSSION
In this real-world population-based analysis of ~2800 individuals treated with SOF/VEL±RBV who were mostly infected with GT1 or GT3, SVR rates were high and ranged from 93.7% for GT3 to 96.4% for GT2. In comparison, SVR12 rates in a pooled meta-analysis of 6 clinical trials ranged from 94.7% for GT3 to 99.4% for GT2 [5]. Although the overall SVR rate in our study was lower for GT3-infected individuals, there was no statistically significant association between GT3 and non-SVR in multivariable analysis.
Our results are similar to the small number of large studies evaluating SOF/VEL effectiveness in real-world settings. In the largest real-world analysis published to date, Belperio et al. evaluated SOF/VEL±RBV among ~4500 individuals with GT2 or GT3 infection in the US Department of Veterans Affairs registry [15]. Similar to our study, effectiveness ranged from 90.7% for GT3 to 93.9% for GT2. In another large but unpublished study of 12 clinical practice cohorts across 8 countries in North America and Europe (n = 4491), the overall SVR was 92.7% [27]. In contrast, SVR was higher (98.5%) in a study of 1319 patients from treatment centers in Italy [17]. Seven other published real-world studies have evaluated SOF/VEL, but most were small, with a combined sample size of <1000 [28, 29].
Comparisons to other real-world studies should be made with caution. “Real-world” is a broad term for describing studies conducted outside of a clinical trial setting, yet there are important differences between studies. Some are not population-based but instead limited to specific treatment centers, registries, or patient populations. Further, some studies use less conservative analytic approaches in which most/all individuals lacking SVR assessment are excluded. For example, the Belperio et al. study was limited to patients receiving medical care through the Veterans Health Administration and excluded GT1-infected patients [15]; the combined North America/Europe analysis excluded individuals with decompensated cirrhosis, previous DAA failure, and RBV added to their regimen [27]; and the Italian study enrolled patients from treatment centers where closer monitoring was provided and excluded those with previous NS5A inhibitor therapy [17]. In comparison with all 3 of these large studies, our analysis was population-based and thus potentially more generalizable to the real-world clinical setting. Given our more real-world setting and conservative approach, it is reassuring that our SVR rates were similarly high as those observed in other studies.
Our conservative analytic approach allowed for greater insight into underlying reasons for actual or potential non-SVR. Just over half of non-SVR cases were nonvirological failures, and about 80% of these lacked SVR assessment and were assumed non-SVR. These individuals would be excluded from per-protocol (PP) analyses. Most non-SVR individuals were categorized as LTFU (treatment completed, last RNA test after treatment initiation was positive, no assessment for SVR). It is reasonable to assume that many of these individuals did not reach SVR, given the positive RNA test and that factors contributing to their loss to follow-up may have also impeded adherence to daily medication (eg, housing instability, substance use, mental health issues). Even for those who did attain SVR, these individuals are still important to characterize, as they may be at higher risk of re-infection due to challenges in remaining connected to the health care system and potentially receiving support to prevent re-infection. Our nonvirological failure rate was lower than the combined analysis of 12 cohorts in North America/Europe, where 80% of non-SVR cases were nonvirological [27]. This may be partly due to their use of SVR12/24 rather than SVR10.
PWID and people with HIV (PWH) had lower SVR after adjustment for other factors. Notably, however, SVR rates for PWID and PWH were higher than 92% for GT2 and GT3 but lower (89% and 84%, respectively) for GT1. Clinical trials have also demonstrated high SVR rates (≥94%) for SOF/VEL among PWID and PWH [30, 31]. However, similar to our analysis and other analyses conducted by our team [32, 33], the large Italian SOF/VEL real-world study found PWID to be associated with non-SVR [17]. Increased odds of non-SVR among PWID and PWH are likely due to social rather than biological factors. Indeed, a particularly high percentage of non-SVR among PWID was due to nonvirological vs virological failure, similar to our previous analyses of other HCV treatments [32, 33]. Of note, our analysis likely underestimates the true SVR rate among PWID, as some of these nonvirological failures may have achieved SVR, and PWID were also overly represented among excluded individuals who had a negative RNA test post–treatment initiation but no SVR assessment. Our findings should not be interpreted as a reason to withhold treatment from these populations, but rather a reminder that optimal scale-up to reach HCV elimination among PWID and PWH will need to be done with additional support measures to prevent LTFU and achieve SVR.
Our study found RBV to be associated with non-SVR among GT3-infected individuals. These findings are similar to other real-world observational SOF/VEL studies suggesting little to no overall benefit with the addition of RBV [15, 17, 34] but seemingly contradict results from clinical trials [5]. The lower SVR among RBV-treated individuals in our study was particularly noticeable for GT3-infected individuals with treatment experience (75% achieved SVR). The contrasting findings between real-world studies and clinical trials may be due to a number of factors, including different patient populations, study designs, and analytic approaches (PP vs ITT). In particular, evaluation of RBV is limited by the observational nature of real-world studies such as ours (eg, RBV is more likely to be prescribed to individuals who are already less likely to achieve SVR), particularly when important confounding variables (cirrhosis, resistance-associated substitutions) may be underidentified due to reliance on administrative data. For example, in our analysis, 80% of RBV-treated individuals had no evidence of cirrhosis (Supplementary Table 4), a key indication for RBV in BC. This finding is more likely to be due to underidentification of cirrhosis (due to reliance on diagnostic codes and lack of fibrosis assessment data) rather than inappropriate prescribing of RBV. As a result, residual confounding by indication may explain the lower SVR with RBV. Further research is needed to better understand the underlying reasons for non-SVR among RBV-treated individuals and the utility of adding this medication in the real world.
GT3 infection, treatment experience, and cirrhosis were not associated with non-SVR in our full multivariable model. In contrast, the large real-world Belperio et al. study found decompensated cirrhosis, FIB-4 >3.25, and treatment experience to be associated with reduced odds of SVR among GT3 patients treated with SOF/VEL or daclatasvir/SOF [15]. Another real-world SOF/VEL study found lower SVR among GT3-infected individuals previously treated with SOF-based regimens [9]. Our ability to detect a difference may have been limited by the small sample size of some of these populations, which, as already discussed, may be an artifact of our data source. Notably, treatment experience was associated with non-SVR in our full and GT3-specific multivariable models when the treatment regimen variable (SOF/VEL+RBV vs SOF/VEL alone) was removed.
Our study has some limitations. Although our analysis was population-based for individuals in BC diagnosed with HCV through the end of 2015 and dispensed SOF/VEL from 2016 to 2018, it did not include individuals who were newly tested and diagnosed from 2016 to 2018. We estimate that about 2000 individuals were newly diagnosed with HCV in this 3-year time frame, but that most would not have received treatment with SOF/VEL by the end of 2018. Our data did not contain any information on clinical fibrosis stage; previous HCV treatments received outside of BC; resistance-associated substitutions; underlying reasons for treatment discontinuation (eg, side effects), LTFU, or RNA testing; or adherence to daily pill-taking among those dispensed a full treatment course. As a result of the latter, we could not estimate the proportion of nonresponse due to suboptimal adherence. Furthermore, we are also not able to tease apart relapse from re-infection, which requires genome sequencing. As most of our variables were based on administrative data, misclassification/underascertainment may have led to residual confounding in regression analyses. Treatment decisions in BC are made at the clinician’s discretion and may differ from other settings, where norms are different, limiting the generalizability of our findings to other jurisdictions (eg, differences in RBV use and GTs). Finally, although ITT and PP are terms commonly used in real-world observational studies, they were originally intended for describing randomized controlled trials [35]. As such, it is important to emphasize that our analysis was observational, and thus confounding could not be completely addressed.
In conclusion, our conservative population-based analysis of SOF/VEL showed high effectiveness in real-world clinical practice in the Canadian province of BC. Our results suggest room for further improvement in supporting individuals to obtain SVR and prevent LTFU, particularly among PWID and PWH. The high proportion of non-SVR due to nonvirological failure demonstrates the importance of a conservative analytic approach when conducting real-world studies.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We acknowledge the assistance of the Provincial Health Services Authority, BC Ministry of Health, and BC Cancer staff involved in data access, procurement, and management. We gratefully acknowledge the residents of British Columbia whose data are integrated in the BC Hepatitis Testers Cohort and whom this work is intended to benefit. We acknowledge and thank all the BC Hepatitis Testers Cohort Team members.
Financial support. This work was supported by the BC Centre for Disease Control and the Canadian Institutes of Health Research (Grant Nos. NHC-348216, PJT-156066, PJT-156117).
Disclaimer. All inferences, opinions, and conclusions drawn in this manuscript are those of the authors and do not reflect the opinions or policies of the British Columbia Ministry of Health and the Data Steward(s).
Potential conflicts of interest. M.K. has received grant funding via his institution from Roche Molecular Systems, Boehringer Ingelheim, Merck, Siemens Healthcare Diagnostics, and Hologic Inc. E.Y. is an investigator of clinical trials sponsored by Gilead Sciences, Abbvie, Merck, Intercept, Allergan, Madrigal and Celgene and he has received honoraria for lectures sponsored by Merck Canada, Gilead Canada and Celgene Canada. A.R. has served as an investigator and/or consultant for AbbVie, Allergen, Assembly, Gilead, Merck, Janssen, Springbanks and Novartis and has received speaker’s grants from AbbVie, Celgene, Novartis, Merck and Gilead. D.C. has received fees for speaking and teaching from Hologic. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. N.J. conceived the analysis presented in this paper. N.J., A.Y., M.A., and M.K. participated in the study design. N.J. guided the statistical analysis, which was performed by S.W. and J.W. J.W. wrote the first draft and incorporated revisions. All authors contributed to the interpretation of results, manuscript preparation, and revisions. All authors read and approved the final manuscript.
Data availability. Data are not publicly available.
References
- 1. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017; 2(3):161–76. [DOI] [PubMed] [Google Scholar]
- 2. Thrift AP, El-Serag HB, Kanwal F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat Rev Gastroenterol Hepatol 2017; 14:122–32. [DOI] [PubMed] [Google Scholar]
- 3. Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci 2006; 3:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Global health sector strategy on viral hepatitis Available at: https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/. Accessed 1 July 2019.
- 5. Ahmed H, Abushouk AI, Attia A, et al. . Safety and efficacy of sofosbuvir plus velpatasvir with or without ribavirin for chronic hepatitis C virus infection: a systematic review and meta-analysis. J Infect Public Health 2018; 11:156–64. [DOI] [PubMed] [Google Scholar]
- 6. Everson GT, Towner WJ, Davis MN, et al. . Sofosbuvir with velpatasvir in treatment-naive noncirrhotic patients with genotype 1 to 6 hepatitis C virus infection: a randomized trial. Ann Intern Med 2015; 163:818–26. [DOI] [PubMed] [Google Scholar]
- 7. Pianko S, Flamm SL, Shiffman ML, et al. . Sofosbuvir plus velpatasvir combination therapy for treatment-experienced patients with genotype 1 or 3 hepatitis C virus infection: a randomized trial. Ann Intern Med 2015; 163:809–17. [DOI] [PubMed] [Google Scholar]
- 8. Curry MP, O’Leary JG, Bzowej N, et al. ; ASTRAL-4 Investigators Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med 2015; 373:2618–28. [DOI] [PubMed] [Google Scholar]
- 9. von Felden J, Vermehren J, Ingiliz P, et al. . High efficacy of sofosbuvir/velpatasvir and impact of baseline resistance-associated substitutions in hepatitis C genotype 3 infection. Aliment Pharmacol Ther 2018; 47:1288–95. [DOI] [PubMed] [Google Scholar]
- 10. Foster GR, Afdhal N, Roberts SK, et al. ; ASTRAL-2 Investigators; ASTRAL-3 Investigators Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015; 373:2608–17. [DOI] [PubMed] [Google Scholar]
- 11. Greig SL. Sofosbuvir/velpatasvir: a review in chronic hepatitis C. Drugs 2016; 76:1567–78. [DOI] [PubMed] [Google Scholar]
- 12. Weisberg IS, Jacobson IM. A pangenotypic, single tablet regimen of sofosbuvir/velpatasvir for the treatment of chronic hepatitis C infection. Expert Opin Pharmacother 2017; 18:535–43. [DOI] [PubMed] [Google Scholar]
- 13. Saeed S, Strumpf EC, Walmsley SL, et al. ; Canadian Co-Infection Cohort Study How generalizable are the results from trials of direct antiviral agents to people coinfected with HIV/HCV in the real world? Clin Infect Dis 2016; 62:919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Backus LI, Belperio PS, Shahoumian TA, et al. . Effectiveness of sofosbuvir-based regimens in genotype 1 and 2 hepatitis C virus infection in 4026 U.S. veterans. Aliment Pharmacol Ther 2015; 42:559–73. [DOI] [PubMed] [Google Scholar]
- 15. Belperio PS, Shahoumian TA, Loomis TP, et al. . Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J Hepatol 2019; 70:15–23. [DOI] [PubMed] [Google Scholar]
- 16. Sadler MD. Editorial: genotype 3 HCV-who still needs ribavirin in a pan-genotypic era? Aliment Pharmacol Ther 2018; 47:1548–9. [DOI] [PubMed] [Google Scholar]
- 17. Mangia A, Piazzolla V, Giannelli A, et al. . SVR12 rates higher than 99% after sofosbuvir/velpatasvir combination in HCV infected patients with F0-F1 fibrosis stage: a real world experience. PLoS One 2019; 14:e0215783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Public Health Agency of Canada. Report on hepatitis B and C in Canada Available at: https://www.canada.ca/en/services/health/publications/diseases-conditions/report-hepatitis-b-c-canada-2016.html. Accessed 1 July 2019.
- 19. Government of British Columbia. More patients to benefit from hepatitis C treatments Available at: https://news.gov.bc.ca/releases/2017HLTH0037-000374. Accessed 1 July 2019.
- 20. Government of British Columbia. Adults with chronic hepatitis C - PharmaCare expanded coverage for adults with chronic hepatitis C infection Available at: https://www2.gov.bc.ca/assets/gov/health/health-drug-coverage/pharmacare/chc_expanded_coverage.pdf. Accessed 1 July 2019.
- 21. Government of British Columbia. Chronic hepatitis C medication now available for all British Columbians Available at: https://news.gov.bc.ca/releases/2018HLTH0017-000387. Accessed 1 July 2019.
- 22. Shah H, Bilodeau M, Burak KW, et al. ; Canadian Association for the Study of the Liver The management of chronic hepatitis C: 2018 guideline update from the Canadian Association for the Study of the Liver. CMAJ 2018; 190:E677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018; 69(2):461–511. [DOI] [PubMed] [Google Scholar]
- 24. AASLD-IDSA HCV Guidance Panel. Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis Off Publ Infect Dis Soc Am 2018; 67(10):1477–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janjua NZ, Kuo M, Chong M, et al. . Assessing hepatitis C burden and treatment effectiveness through the British Columbia Hepatitis Testers Cohort (BC-HTC): design and characteristics of linked and unlinked participants. PLoS One 2016; 11:e0150176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Backus LI, Belperio PS, Shahoumian TA, et al. . Real-world effectiveness of ledipasvir/sofosbuvir in 4365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology 2016; 64:405–14. [DOI] [PubMed] [Google Scholar]
- 27. Mangia A, Milligan S, Khalili M, et al. . Global real world evidence of sofosbuvir/velpatasvir as a simple, effective regimen for the treatment of chronic hepatitis C: integrated analysis of 12 clinical practice cohorts. Paper presented at: European Association for the Study of the Liver Conference; 10–14 April 2019; Vienna, Austria. [Google Scholar]
- 28. Pisaturo M, Russo A, Onorato L, Coppola N. Efficacy of 12-weeks velpatasvir plus sofosbuvir-based regimen in HCV-naive subjects with mild fibrosis: a meta-analysis. Acta Biomed 2019; 90:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buggisch P, Wursthorn K, Stoehr A, et al. . Real-world effectiveness and safety of sofosbuvir/velpatasvir and ledipasvir/sofosbuvir hepatitis C treatment in a single centre in Germany. PLoS One 2019; 14:e0214795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wyles D, Bräu N, Kottilil S, et al. ; ASTRAL-5 Investigators Sofosbuvir and velpatasvir for the treatment of hepatitis C virus in patients coinfected with human immunodeficiency virus type 1: an open-label, phase 3 study. Clin Infect Dis 2017; 65:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grebely J, Dalgard O, Conway B, et al. ; SIMPLIFY Study Group Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol 2018; 3:153–61. [DOI] [PubMed] [Google Scholar]
- 32. Darvishian M, Wong S, Binka M, et al. . Loss to follow‐up: a significant barrier in the treatment cascade with direct‐acting therapies. J Viral Hepat 2020; 27(3):243–60. [DOI] [PubMed] [Google Scholar]
- 33. Janjua NZ, Darvishian M, Wong S, et al. ; British Columbia Hepatitis Testers Cohort Team Effectiveness of ledipasvir/sofosbuvir and sofosbuvir/velpatasvir in people who inject drugs and/or those in opioid agonist therapy. Hepatol Commun 2019; 3:478–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Landis CS, Sulkowski MS, Real N, et al. . Safety and efficacy of velpatasvir and sofosbuvir-based regimens for the treatment of HCV genotype 1–6: results of the HCV TARGET Study. Paper presented at: American Association for the Study of Liver Diseases Conference; 20–24 October 2017; Washington, DC. [Google Scholar]
- 35. Ojha RP, Steyerberg EW. Real-world data on antiviral treatments for hepatitis C virus infections: can we define intention to treat or per protocol analyses? J Hepatol 2018; 69:551–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.