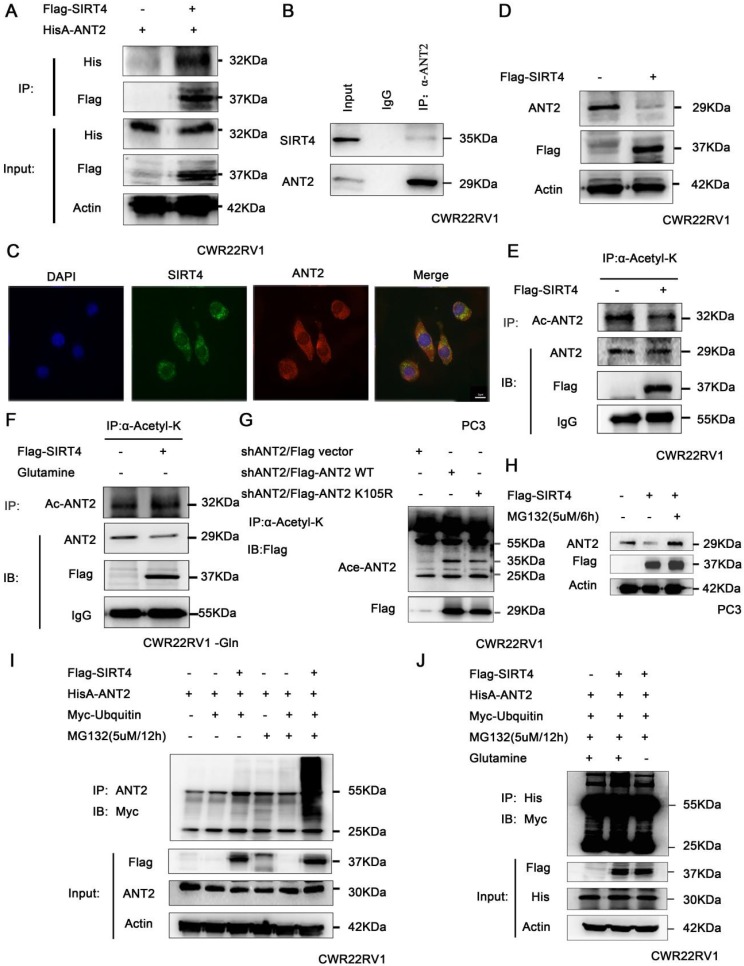
Figure 3.
SIRT4 promotes the ubiquitination degradation of ANT2 depending on its deacetylation activity. (A) HEK293 cells expressing HisA-ANT2 were co-transfected with or without Flag-tagged SIRT4, and (B) the endogenous SIRT4 and ANT2 were evaluated in CWR22RV1 cells. The SIRT4-ANT2 interaction was identified through immunoprecipitation and western blot. (C) The co-localization of endogenous SIRT4 (red), ANT2 (green), and the nuclei (DAPI blue). The merged images with the nucleus are shown, as indicated. Original magnification: ×40. (D) CWR22RV1 cells were transfected with Flag-tagged SIRT4. The protein expression levels were determined by western blot. (E-F) CWR22RV1 cells were transfected with Flag-tagged SIRT4, with or without glutamine, the broad-spectrum acetylated antibody was enriched for acetylated protein, and the Ac-ANT2 protein expression levels were determined by immunoprecipitation and western blot. (G) The indicated ANT2 residues were mutated, as indicated (K105R), and the resulting proteins analyzed via immunoprecipitation and western blot. (H-I) Cells that overexpressed SIRT4 were incubated with a medium containing 5 μM of MG132 for 12 hours. The ANT2 expression levels were determined by western blotting and immunoprecipitation with the indicated antibodies. (J) CWR22RV1 cells that were transfected with the indicated constructs were exposed to 5 μM of MG132 for 12 hours in culture medium, with or without glutamine. Western blot was performed with the indicated antibodies.