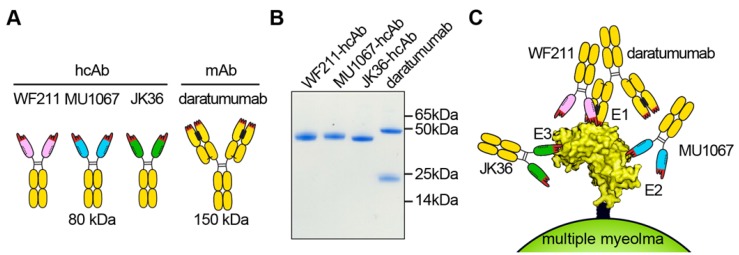
Figure 1.
Structure, purity, and binding epitopes of CD38-specific antibody constructs. (A) Scheme of heavy chain antibodies (80 kDa). The single-domain antibody fragments (nanobodies) are indicated in pink (WF211), blue (MU1067), and green (JK36). The hinge and Fc-domains of human IgG1 are indicated in black and yellow, respectively. The three CDR loops of the antigen-binding paratope are indicated in red. Conventional antibody daratumumab (150 kDa) is indicated in yellow (heavy chains and light chains). Hydrophobic patches at the interface between the VH and VL domains are indicated in black. The corresponding hydrophilic patch in the nanobodies is indicated by dashed lines. (B) Coomassie-staining of an SDS-PAGE under reducing conditions with CD38-specific heavy chain antibodies WF211-hcAb, MU1067-hcAb, JK36-hcAb and daratumumab verifies the purity and integrity of the constructs used in this study. Note that daratumumab consists of two heavy chains (50 kDa) and two light chains (25 kDa). The hcAbs consist of two identical heavy chains (42 kDa). Each lane contains 2 µg of the indicated construct. (C) The three hcAbs recognize three distinct epitopes of CD38. WF211-hcAb recognizes epitope 1 (E1), MU1067-hcAb recognizes epitope 2 (E2), and JK36-hcAb recognizes epitope 3 (E3). The binding epitope E1 of WF211-hcAb overlaps with the epitope of daratumumab.