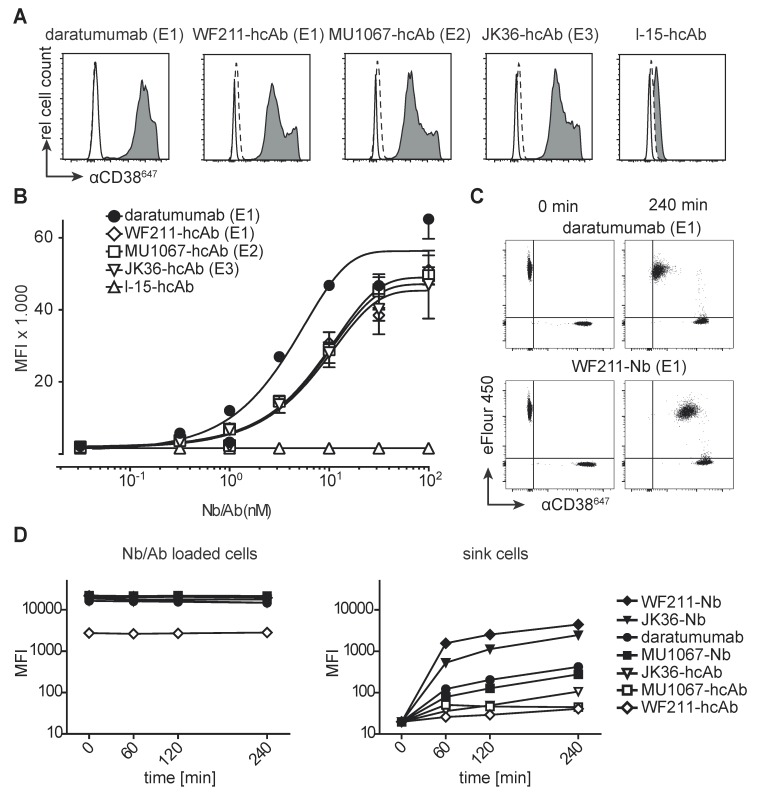
Figure 2.
Binding of CD38-specific hcAbs to lymphoma cells. (A) Untransfected CD38-negative parental Yac-1 cells (open histograms) and their counterparts stably transfected with human CD38 (grey histograms) were stained with CD38-specific hcAbs WF211-hcAb, MU1067-hcAb, JK36-hcAb, isotype control (l-15-hcAb), or daratumumab. Binding was detected with a PE-conjugated human IgG-specific secondary antibody. Control stainings were performed with the PE-conjugated secondary antibody alone (dashed lines). Epitope specificities of CD38-specific antibodies are given in parentheses. (B) Titration analysis of the binding of CD38-specific hcAbs to CD38-transfected Yac-1 cells. Cells were incubated with serial dilutions of CD38-specific hcAbs, isotype control (l-15-hcAb), or daratumumab. Binding was detected with a PE-conjugated human IgG-specific secondary antibody. Data represent mean ± SD from three independent experiments. MFI, mean fluorescence intensity. (C) Representative dot plots and (D) changes in mean fluorescence intensity over time of the dissociation of fluorochrome-conjugated monovalent nanobodies and bivalent hcAbs from CD38-transfected Yac-1 cells. Cells were incubated with excess (100 nM) Alexa647-conjugated nanobodies, hcAbs or daratumumab for 30 min at 4°C. Cells were washed three times and then monitored for loss of cell-associated fluorescence over time at RT. An aliquot of CD38-expressing Yac-1 cells that had been labeled with the cell-tracking dye eFluor 450 was added at t = 0 as a sink for the dissociated antibody constructs.