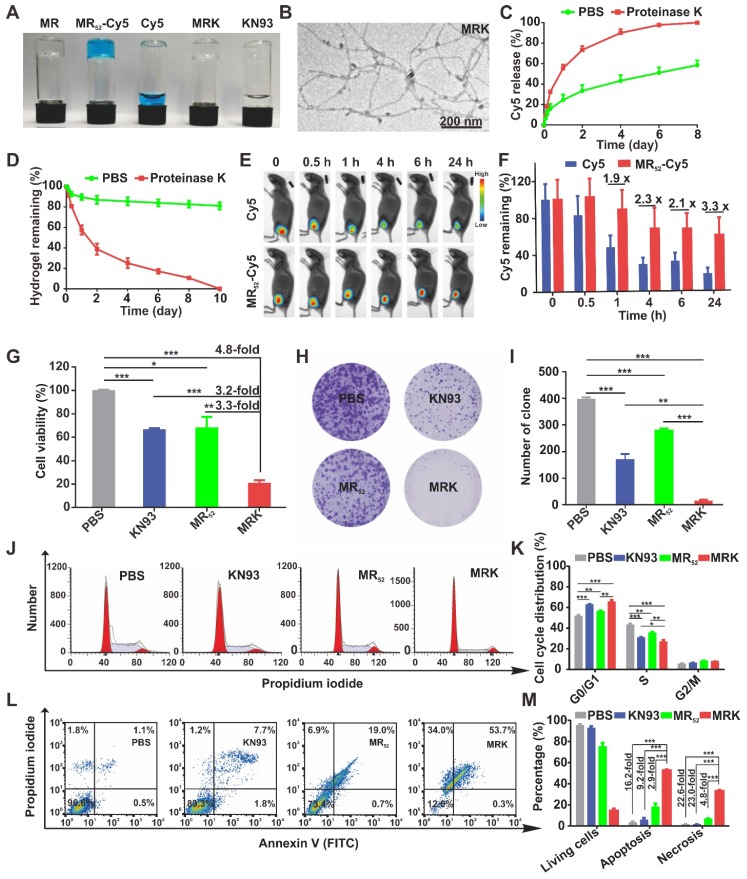
Figure 1.
Synthesis, characterization, and in vitro antitumor effects of the MRK hydrogel. (A) Photographs of MR52 hydrogels or MR52 hydrogels loaded with various agents. (B) The representative TEM image of the MRK hydrogel. Scale bar, 200 nm. (C) Comparison of the release rate of Cy5 from the MR52-Cy5 hydrogel in the presence or absence of proteinase K. (D) Comparison of the MR52 hydrogel release rate in the presence or absence of proteinase K. (E) The NIR fluorescence imaging analysis of the distribution of the MR52-Cy5 hydrogel and free Cy5 in vivo. The subcutaneous implantation was performed at the indicated time points. (F) Quantitative data of the NIR fluorescence imaging results, Data are presented as the mean ± SEM (n = 3). (G) Cell viability measurement using the CCK-8 assay. (H, I) Clone formation assay (left panel: representative images of cell clones treated with PBS, KN93, and MR52, or MRK hydrogel; right panel: quantification of cell clones; n = 3). (J) Representative images of the cell cycle induced by PBS, KN93, MR52, or MRK hydrogel per the flow cytometry analyses. (K) Quantitative data from (J). Data are presented as the mean ± SEM (n = 4). (L-M) Flow cytometry analyses of cell apoptosis induced by PBS, KN93, MR52, or MRK hydrogel. Data are presented as the mean ± SEM (n = 3).