Fig. 4.
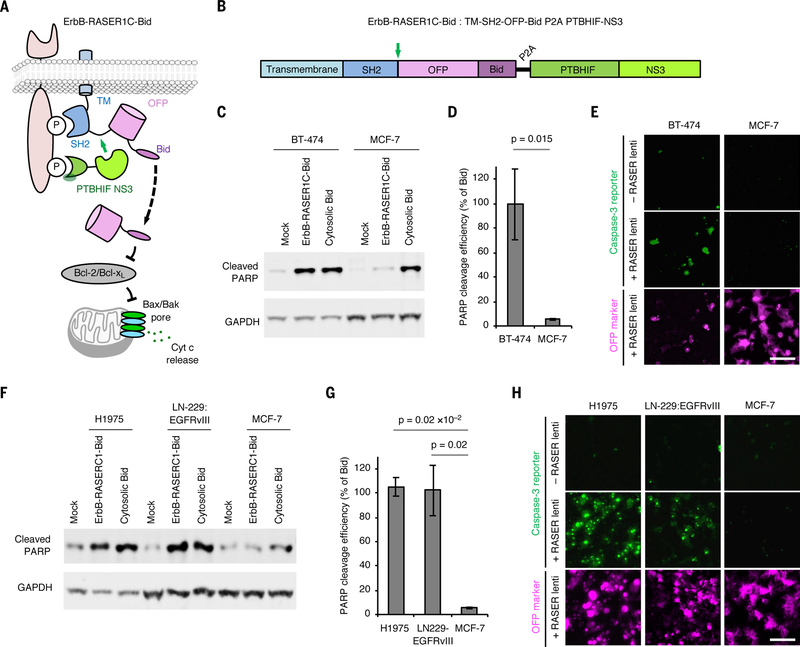
RASER can be programmed to induce apoptosis selectively in ErbB-driven cancer cells. (A) Schematic description of the ErbB-RASER1C-Bid system. OFP-Bid will be released in the presence of constitutive ErbB signaling. (B) ErbB-RASER1C-Bid is a single transcription unit encoding both substrate and protease components that can be expressed at a constant ratio by transfection with a single plasmid or transduction by a single virus. Green arrow, protease cleavage site to release cargo. (C) BT-474 cells which overexpress ErbB2 and MCF7 cells with normal ErbB levels were transfected with the ErbB-RASER1C-Bid construct. After 16 h of protein expression, cells were lysed for immunoblotting to detect cleaved PARP and GAPDH. (D) Quantitation of cleaved PARP levels in immunoblots. Error bars represent s.e.m. of three biological replicates. The increased RASER output in BT-474 cells compared to MCF-7 cells was statistically significant by one-tailed unpaired t test. (E) ErbB-RASER1C-Bid virus infection efficiently induced apoptosis in BT-474 cells but not MCF-7 cells. Apoptosis was visually assessed using a fluorescent caspase-3 activity indicator. Scale bar, 100 μm. (F) Apoptosis inductivity of ErbB-RASER1C-Bid is generalizable in multiple ErbB1 hyperactive cancer cell lines. H1975, LN-229:EGFRvIII and MCF-7 cells were tested in a same method to (B). (G) Quantification of cleaved PARP level, calculated as in (C). (H) Infection with virus expressing ErbB-RASER1C-Bid induced apoptosis in H1975 and LN-229:EGFRvIII cells, which express hyperactive ErbB1 mutants, but not MCF-7 cells which does not express hyperactive ErbB. Apoptosis was visually assessed using a fluorescent caspase-3 activity indicator. Scale bar, 100 μm.