Figure 4.
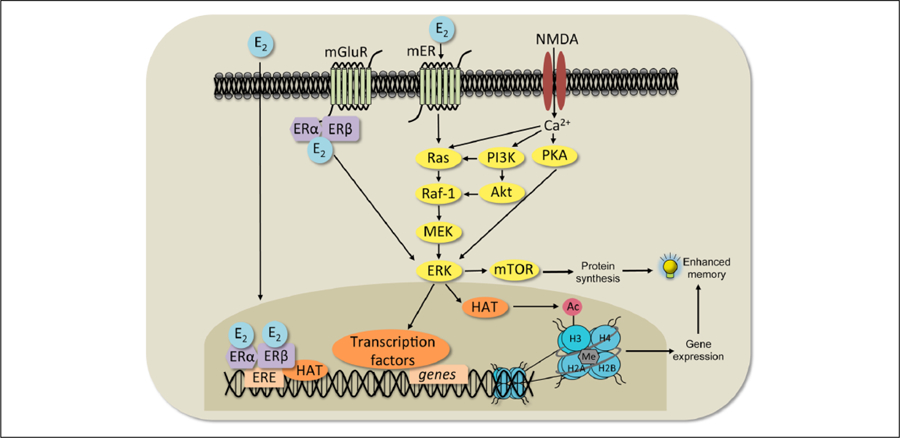
Classical and non-classical 17β-estradiol (E2) signaling mechanisms. In the classical response (left), E2 binds ERα and ERβ, which then translocate into the nucleus, bind to the estrogen response element (ERE) on DNA, and interact with co-regulatory proteins (including histone acetyltransferases, HAT) to influence transcription. In a non-classical response (center), E2 may affect cell signaling in several ways. It can bind to ERs that interact with metabotropic glutamate receptors (mGluRs) at the membrane and activate extracellular regulated kinase (ERK) signaling. E2 can also interact with NMDA receptors and membrane-bound ERs (mER) to activate the protein kinase A (PKA), phosphoinositol-3-kinase (PI3K), and mammalian target of rapamycin (mTOR) signaling pathways. mTOR signaling regulates the protein synthesis necessary for memory formation. Activation of ERK increases histone H3 acetylation (Ac). Both H3 acetylation and DNA methylation (Me) are necessary for E2 to enhance memory consolidation. Adapted with permission from Fortress and Frick (2014).
