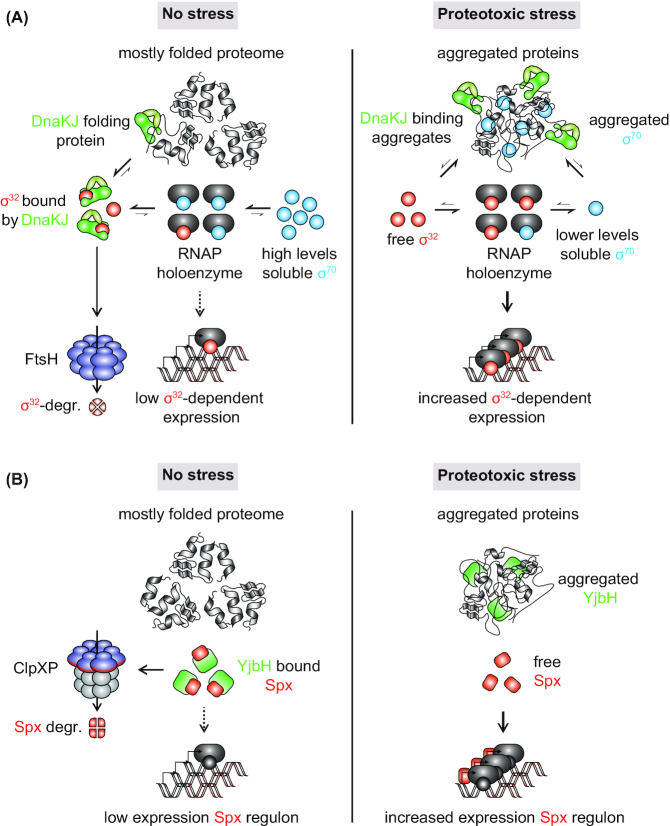
Figure 6.
Protein aggregation in stress adaptation. (A), Possible role of σ70 aggregation in the regulation of the E. coli heat shock response. In the absence of stress, free DnaKJ (GrpE not shown) will bind to the heat shock sigma factor σ32 and facilitate its degradation by the membrane-bound protease FtsH (contribution of SRP to σ32 regulation not shown). High levels of the housekeeping sigma factor σ70 could outcompete residual free σ32 for binding to the RNA polymerase (RNAP), further inhibiting inappropriate heat shock response induction. During heat shock, DnaKJ will largely relocalize to aggregating protein and liberate σ32. The thermosensitive σ70 will aggregate, potentially reducing the levels of soluble molecules capable of competing with σ32 and further enhancing heat shock response induction. (B), Protein aggregation in the regulation of the B. subtilis heat and oxidative stress response. In the absence of stress, levels of the heat and oxidative stress master regulator Spx are kept low through YjbH adaptor-mediated degradation by the protease ClpXP. Aggregation induced through heat and oxidative stress induces co-aggregation of YjbH. Liberated Spx can induce the expression of stress-adaptive genes and the repression of proliferative genes.