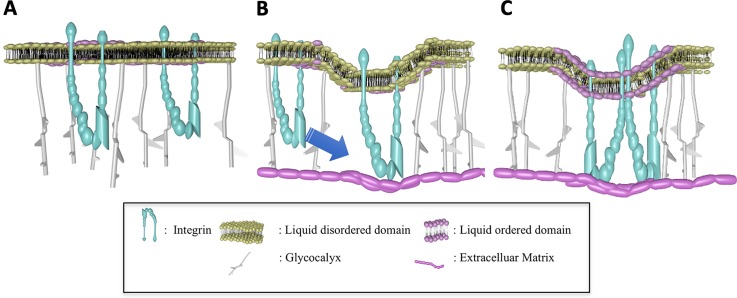
FIG. 2.
Integrin adhesion at sites devoid of glycocalyx with integrin catch bonds supported by elasticity of glycocalyx. A. Integrins exist as monomers in liquid-ordered domains with glycocalyx present uniformly on the cell surface. B. Random defects (or induced defects) in the glycocalyx spacing, thermally induced bending of the membrane, and applied force on the abluminal surface, bias the membrane to close approximation with extracellular matrix molecules. C. Once an integrin molecule is adhered, the bent membrane provides an avenue for additional adhesion, with the glycocalyx providing some of the counter forces of adhesion necessary to maintain catch bonds.