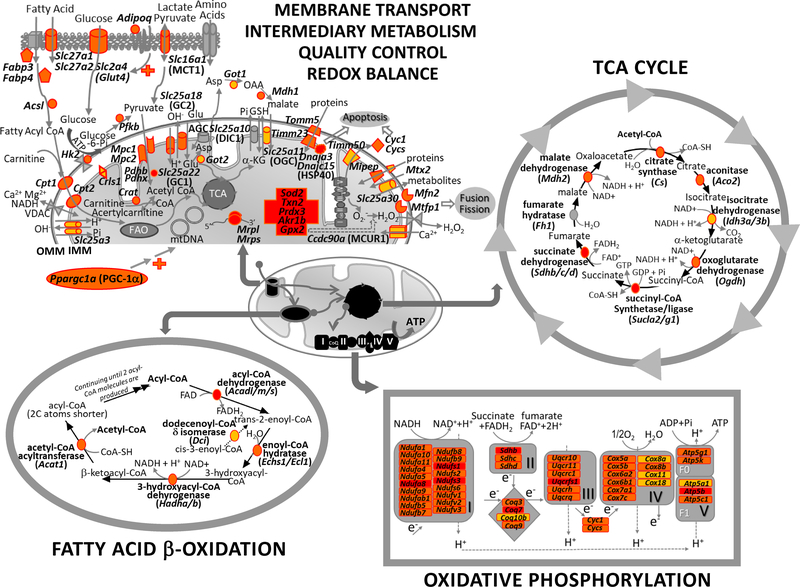
Fig 2. Pulmonary NRF2 effectors known to be involved in mitochondrial bioenergetics and biogenesis.
NRF2-dependently regulated lung genes involved in mitochondrial biogenesis and bioenergetics were determined in a murine study with a NRF2 agonist sulforaphane (Cho et al. 2019). Highlighted proteins in color are encoded from sulforaphane-induced genes in Nrf2-deficient mice but not in Nrf2-sufficient mice at normal physiological condition (in orange), after hyperoxia exposure (an experimental model of acute lung injury, in yellow), or under both conditions (in red). Most of these protein-encoding genes are found to possess potential antioxidant response elements in their upstream region (Selected list in Table 2). In addition to mitochondrial redox balance through reactive oxygen species quenching and protein S-glutathionylation, NRF2 may enhance cellular entry of nutrients and mitochondrial entry of intermediary metabolites by transcriptional activation of plasma and mitochondrial membrane transporters to increase substrates for oxidative phosphorylation and ATP production. NRF2 may also maintain the healthy pool of mitochondria through quality control by induction of genes involved in biogenesis, fusion, fission, mitophagy, and apoptosis. Most importantly, NRF2 may directly induce expression of the genes encoding enzymes for fatty acid β-oxidation (FAO), tricarboxylic acid (TCA) cycle, and oxidative phosphorylation. Overall, NRF2 may exert its action in various cells such as type 2 pneumocytes, bronchial epithelial cells, smooth muscle cells, and endothelial cells to defend airway from pathogenesis. IMM=inner mitochondrial membrane. OMM=outer mitochondrial membrane. Acsl, acyl-CoA synthetase long-chain family member; adipoq, adiponectin, C1Q and collagen domain containing; ADIPOR, adiponectin receptor; AGC, aspartate-glutamate carrier; Aldh, aldehyde dehydrogenase; Asp, aspartic acid; Atp, ATP synthase, H+ transporting, mitochondrial; Ccdc90a, coiled-coil domain containing 90A or mitochondrial calcium uniporter regulator 1 (Mcur1); CoA, coenzyme A; Coq, coenzyme Q; Cox, cytochrome c oxidase subunit; Cpt, carnitine palmitoyltransferase; Crat, carnitine O-acetyltransferase; Crd1, cardiolipin synthase; CYC1, cytochrome cl; CYCS, cytochrome c, somatic; Dic1, mitochondrial dicarboxylate transporter; Dnaj, DnaJ heat shock protein family; Fabp, fatty acid binding protein; GC, glutamate carrier; Glu, glutamic acid; Glut4, glucose transporter type 4; Got, glutamic-oxaloacetic transaminase; GSH, glutathione; Gpx2, glutathione peroxidase 2; Hk2, hexokinase 2; HSP40, heat shock protein 40; α-KG, α-ketoglutarate; MCT1, monocarboxylate transporter 1; MCUR1, mitochondrial calcium uniporter regulator 1; Mdh1, malate dehydrogenase 1; Mfn2, mitofusin 2; Mipep, mitochondrial intermediate peptidase; Mpc, mitochondrial pyruvate carrier; Mrpl, mitochondrial ribosomal protein L; Mrps, mitochondrial ribosomal protein S; mtDNA, mitochondrial DNA; Mtfp1, mitochondrial fission process 1; Mtx2, Metaxin 2; Nduf, NADH dehydrogenase (ubiquinone); OGC, oxoglutarate carrier; OAA, oxaloacetate; Pdh, pyruvate dehydrogenase; Pfkb, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase; Ppargc1a, peroxisome proliferative activated receptor, gamma, coactivator 1 α; Prdx3, peroxiredoxin 3; Sdh, succinate dehydrogenase complex; Slc, solute carrier family; Sod2, superoxide dismutase 2; Timm, translocase of IMM; Tomm5, translocase of outer mitochondrial membrane 5; Txn2, thioredoxin 2; Uqcr, ubiquinol-cytochrome c reductase, complex. VDAC, voltage dependent anion channel.