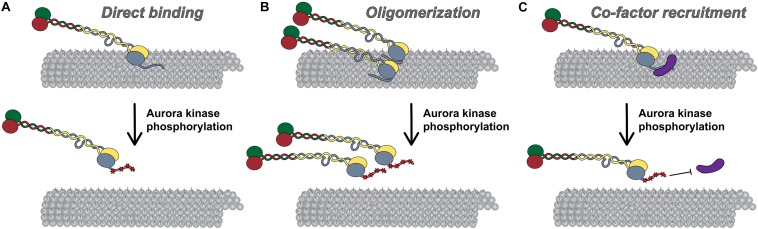
FIGURE 3.
Models for Hec1 tail domain function. (A) Direct microtubule binding. In this model, the tail domain directly interacts with the microtubule lattice to increase CH-domain-mediated NDC80 complex-microtubule interactions. Phosphorylation of the Hec1 tail reduces the positive charge of the tail domain and as a result, reduces the affinity of NDC80 complexes for the negatively charged microtubule lattice. (B) Oligomerization. In this model, a dephosphorylated tail domain functions to oligomerize adjacent NDC80 complexes, which promotes high affinity NDC80-complex-microtubule binding. Upon phosphorylation of the tail domain, complex oligomerization is no longer favored, possibly due to a decrease in affinity of a phosphorylated tail domain for a negatively charged region within the CH domain of Hec1. (C) Co-factor recruitment. In this model, a dephosphorylated Hec1 tail domain recruits kinetochore-associated microtubule binding proteins or protein complexes to promote high affinity NDC80 complex-microtubule binding. In contrast, a phosphorylated tail domain restricts co-factor recruitment. As discussed in the text, these models are not mutually exclusive.