Abstract
This clinical case report describes the simultaneous development of an acute myocardial infarction, stroke, and a massive pulmonary thromboembolism in a 44-year-old patient — a carrier of the thrombophilia gene polymorphisms: MTHFR C677T, А1298C, PAI-1 4G/5G, ITGA2 C807T. Multifocal thrombosis was probably due to the initial congenital deficiency of anticoagulants, accompanied by a decrease in antithrombin III and protein C, against the background of their critical consumption in cascade thrombosis, in combination with the carrier of polymorphisms of moderate and low thrombogenic risk. This case is unique in that there is usually a tendency toward clinical thrombosis when the level of antithrombin III is less than 70%. Such patients develop thrombosis at a younger age, and by the age of 35–40 years usually have a verified diagnosis of extremely high-risk hereditary thrombophilia. In this case, multifocal thrombosis was accompanied by critically low values of anticoagulants: antithrombin III — 3.4%, and protein C — 36.8%. The patient had suffered from epilepsy since childhood and took anticonvulsant drugs that increase the deficit of active folic acid and can lead to hyperhomocysteinemia, which in this case, against the background of an innate decrease in the activity of methyltetrahydrofolate reductase, could have aggravated the situation.
<Learning objective: To focus on the possibility of the manifestation of multifocal thrombosis in congenital thrombophilia in adulthood against the background of critically low values of anticoagulants — antithrombin III and protein C.>
Keywords: ST-elevation myocardial infarction, Stroke, Pulmonary embolism, Inherited thrombophilia, Acquired thrombophilia, Multifocal thrombosis
Introduction
Thrombosis is a common pathology underlying ischemic heart disease, ischemic stroke, and venous thromboembolism [1]. These pathologies remain an unresolved problem for modern medicine. The presence of acute thrombosis requires urgent remedial measures, as well as identifying the underlying causes of the pathology in order to prevent recurrence. A diagnostic search depends on the location, the prevalence of thrombosis, the age and sex of the patient, and associated diseases and risk factors.
When arterial and/or venous thrombosis arises at a young age, among other things, it is necessary to exclude hereditary and acquired thrombophilia. It is known that hereditary thrombophilia cannot be considered as the only cause of thrombotic diseases. However, this is dangerous because of the risk of thrombosis becoming more significant when exposed to external contributory factors [2].
Case report
The patient, 44 years old, complaining of angina pains, dizziness, and mental confusion, was admitted to the intensive care unit (ICU) diagnosed with “acute coronary syndrome”.
Medical history: he had suffered a birth trauma, and had been suffering from epilepsy since the age of 10 years, and was under constant medication — taking oxcarbazepine, pagluferalum®-3 (Moscow Pharmaceutical Factory (Russia), contains phenobarbital + bromisoval + calcium gluconate + caffeine + papaverine). He had been hospitalized a month previously with double pneumonia, and as a result was on a ventilator for 5 days. The patient had no previous anginal pain, myocardial infarction, or stroke, and stated that there was no history of any vascular disease in the family.
When he was admitted his condition was extremely acute —sopor, cyanosis, bilateral diffuse wheezing, blood pressure 85/45 mm Hg, and pulse 100 bpm. The patient’s breaths were 19–22 per minute, SpO2 = 88%., Killip IV, no edema, and body mass index (BMI) = 41 kg/m2. Electrocardiography results: sinus rhythm, 1st degree atrioventricular block, transitory partial right bundle branch block, ST-elevation in II, III, aVF, and ST-depression in I, aVL, and V2–V6.
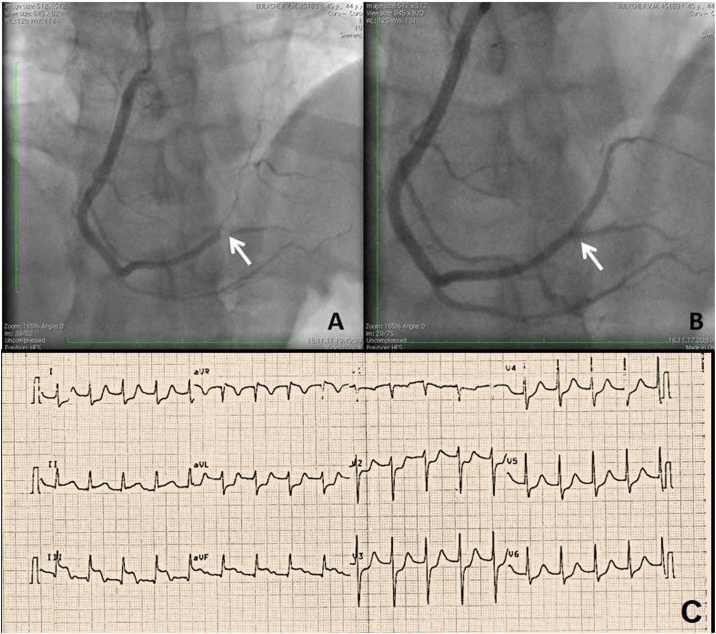
The patient underwent orotracheal intubation with transfer to mechanical ventilation and was assigned dopamine inotropic support 5–6 mcg/kg/min. The patient’s troponin level was 0.35 μg/l (normal range 0–0.1 μg/l, hereinafter the normal range is shown in brackets). In view of this clinical picture, the electrocardiogram result, and the increased level of troponin, an emergency coronary angiography was carried out. An occlusion of the posterior lateral branch of the right coronary artery was discovered, and a balloon angioplasty with stenting was performed (Fig. 1).
Fig. 1.
(А) Occlusion of the right coronary artery — distal third, at the site of bifurcation of the posterior lateral branch and posterior interventricular branch. (B) Stent in the posterior lateral branch of the right coronary artery. Successful recanalization. (C) Electrocardiography results: sinus rhythm, 1st degree atrioventricular block, transitory partial right bundle branch block, ST-elevation in II, III, aVF, and ST-depression in I, aVL, and V2-V6.
A general blood test showed the hemoglobin level at 146 g/l, thrombocytopenia - 132*109/l, and leukocytosis −14.20*109/l. A general urine test showed proteinuria at 0.3 g/l. Blood chemistry analysis showed: aspartate aminotransferase - 138 IU/l, alanine aminotransferase - 118 IU/l, lactate dehydrogenase (LDH) - 1200 IU/l, creatinine - 209 μmol/l, cholesterol – 3.1 mmol/l, triglyceride - 1.46 mmol/l, low-density lipoprotein cholesterol – 1.74 mmol/l, high-density lipoprotein cholesterol - 0.7 mmol/l. A prone position antero-posterior projection chest X-ray revealed no pathology.
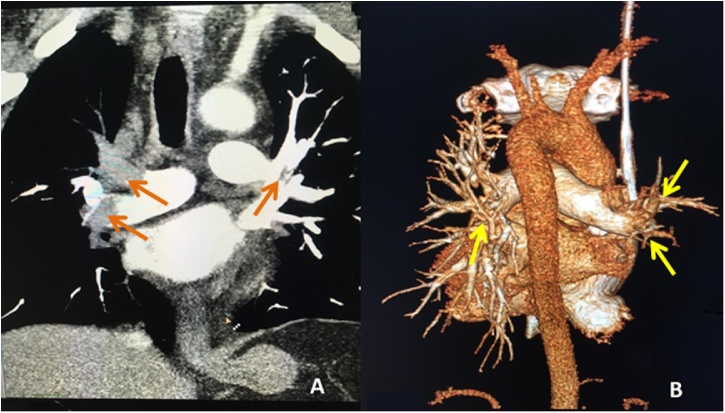
After 24 h LDH - 8244 IU/l, creatinine - 218 μmol/l, and troponin - 7.1 μg/l were observed. The international normalized ratio (INR) was 1.37, the activated partial thromboplastin time (APTT) was 40.4 s, D-dimer level was 46500 ng/ml (normal 64.0–550.0 ng/ml). Right ventricular dilatation with end diastolic diameter 58 mm, systolic pulmonary artery pressure - 70 mmHg, аkinesis of the basal and middle segments of the lower wall of the left ventricle, and asynchronous movement of the interventricular septum were found on echocardiography. In patients with an open oval window thromboembolism of cerebral vessels on the background of pulmonary embolism can occur. The present patient had no data for the presence of an open oval window according to the echocardiography results. Computer angiopulmonography revealed a massive pulmonary embolism (Fig. 2). Bilateral occlusive thrombosis of the posterior tibial, sural, popliteal vein, and superficial femoral veins to the common femoral vein, without flotation, were revealed with ultrasound.
Fig. 2.
Multislice computed tomography – angiography. (А) Аrrows indicate obstructing blood clots in the lumen of the right main pulmonary artery and a parietal clot in the lumen of the left main pulmonary artery. A sharp depletion of vascular pattern in the pulmonary tissue, more on the right. (B) 3D reconstruction. Arrows indicate “breaks” of vessels in places of blockage by thrombotic masses. A sharp depletion of vascular pattern in the pulmonary tissue, more on the right.
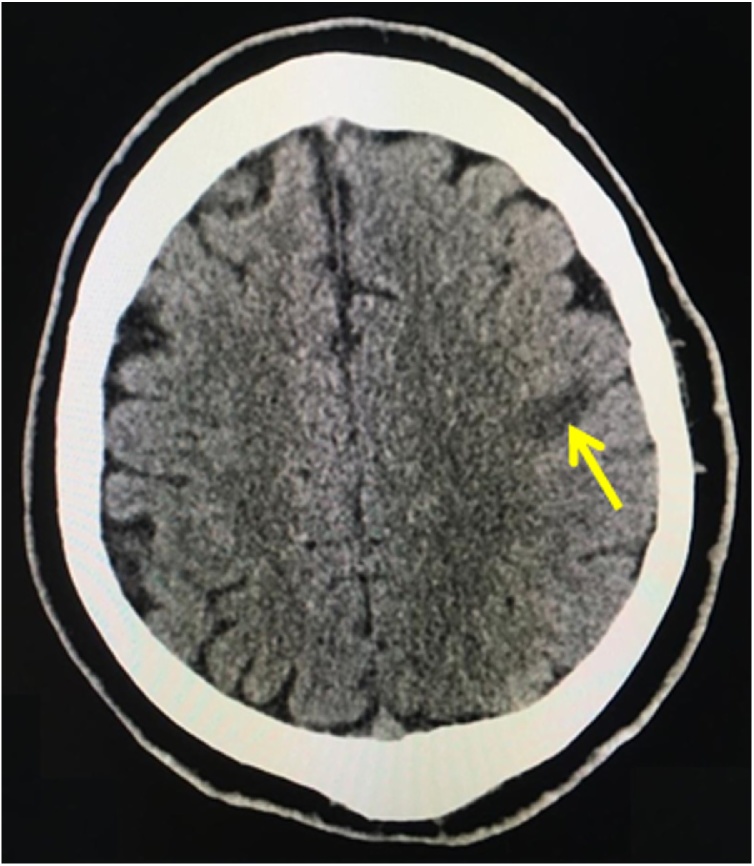
After 48 h, the patient displayed dysarthria. Brain computer tomography revealed signs of ischemic stroke in the pool of terminal branches of the left middle cerebral artery (Fig. 3).
Fig. 3.
Brain computed tomography: in the left hemisphere the ischemic area measuring 19 × 12 × 12 mm is visualized in the projection of the convexital left parietal lobe in the projection of the postcentral gyrus. Brain matter in other areas are without focal density changes. Conclusion: signs of stroke of the ischemic type in the pool of terminal branches of the left middle cerebral artery.
Аntithrombin III (AT III) - 3.4% (80–120%), protein C - 36.8% (70–140%) and folic acid - 1.8 ng/ml (3.1–20.5 ng/ml) were found in the coagulogram.
Given the presence of multisystem thrombosis and the young age of the patient, a molecular genetic study was conducted. It was found that the patient was a carrier of a polymorphism of thrombophilia genes: methyltetrahydrofolate reductase mutation (MTHFR) C677T and A1298C, mutation plasminogen activator inhibitor 1 (PAI-1) 4G/5G, and mutation of platelet collagen receptors integrin alpha-2 (ITGA2) C807T.
Certain conditions were ruled out as result of the differential diagnostics: systemic lupus erythematosus and antiphospholipid syndrome (lupus anticoagulant, antibodies to beta2-glycoprotein, and antibodies to cardiolipin were negative), thrombotic thrombocytopenic purpura (schizocytosis in the blood was not detected, the determination of ADAMTS13 was not possible), cancer pathology, and other hereditary thrombophilia [mutations of factor V Leiden, factor VII G10976A, factor XIII F13A1, integrin beta-3 T1565C, prothrombin G20210A, fibrinogen not detected, fibrinogen level - 2.9 g/l (1.8–3.5 g/l)].
The patient was treated in the intensive care unit for 21 days: replenishment of AT III, anticoagulant therapy with dabigatran etexilate, dual antiplatelet therapy with acetylsalicylic acid and clopidogrel were carried out; folic acid was prescribed at 10 mg daily, bisoprolol 5 mg daily, and enalapril 20 mg daily. Forty five days after admission, the patient was discharged from the hospital, respiratory failure fully regressed, heart failure was compensated, and a minimal neurological deficit remained in the form of dysarthria.
It is known that after discharge the patient did not control his blood pressure, and took only dabigatran etexilate - 150 mg daily, folic acid - 5 mg daily, and an anticonvulsant therapy. Ten months after his discharge, the patient suffered a second ischemic stroke in the pool of the right middle cerebral artery. At the present time (2 months after the described hospitalization) the state of his health is satisfactory; post-stroke dysarthria still present, BMI = 46 kg/m2, pulse 80 bpm, blood pressure 140/90 mmHg, periodic increase of blood pressure to 200/110 mmHg. He is now recommended to take dabigatran etexilate – 150 mg*2 daily, folic acid - 5 mg daily, bisoprolol - 5 mg daily, enalapril - 20 mg daily, atorvastatin - 40 mg daily, and oxcarbazepine, pagluferal-3.
Discussion
The patient was found to have the simultaneous development of acute myocardial infarction, acute cerebrovascular accident, and pulmonary embolism, which is extremely rare. The clinical picture and results of the coagulogram allow us to speak with confidence about the absence of disseminated intravascular coagulation syndrome in the patient. The thrombophilia was not associated with cancer. Given the age of the patient, his personal history, the prevalence and severity of his thrombosis, the critical decrease in the level of AT III and protein C, the patient was examined for the presence of hereditary thrombophilia.
The combination of genotypes MTHFR C677T and A1298C increases the likelihood of neural tube defects in the fetus, which may result in the patient developing epilepsy in childhood [3]. According to researchers, the prevalence of mutations in the gene MTHFR C677T and A1298C is 3.67%. Of these, 28.7% have deep venous thrombosis, 12.9% - stroke, 0.07% - coronary heart disease [4]. In carriers of the mutation MTHFR C677T there is a decrease in the activity of the enzyme methyltetrohydrophthalate reductase, which in combination with taking anticonvulsant drugs that violate the absorption of folic acid in the intestine, leads to a decrease in the active form of folic acid and leads to hyperhomocysteinemia. The level of homocysteine in the patient’s blood at the time of this hospitalization was not determined for technical reasons. The ITGA2 C807T mutation leads to a change in platelet receptors for collagen and an increase in the platelet adhesion rate. Various researchers have shown the association of the presence of allele T in the gene ITGA2 C807T with the risk of myocardial infarction, ischemic stroke, and thromboembolism, especially at a young age. Mutation PAI-1 4G/5G leads to a decrease in blood fibrinolytic activity. A meta-analysis of 9 studies showed a 20% increased risk of myocardial infarction attributed to the 4G/4G genotype [5].
Also, the patient has a congenital deficiency of natural anticoagulants-AT III and protein C. The prevalence of protein C deficiency in a healthy population is 0.2–0.5%, in patients with venous thromboembolism (VTE) – 2–5% [6]. The risk of primary VTE in individuals with protein C deficiency is 0.4–2.3% per year, the risk of VTE recurrence is 1.8% per year [7]. The prevalence of protein C deficiency in patients with ischemic stroke varies from 4% to 21% [8]. The prevalence of AT III deficiency in a healthy population is 0.02–0.04%, in patients with VTE-1-2% [6]. VTE in patients with AT III deficiency usually occurs as deep vein thrombosis of the legs and arms and pulmonary embolism, but can also occur in unusual locations such as cerebral or sinus, mesenteric, portal, hepatic, renal, and reticular veins. Clinically significant reduction in AT III is less than 70% [9]. In this case, multifocal thrombosis was accompanied by critically low values of anticoagulants: AT III-3.4%, protein C-36.8%. It is impossible to say what was primary, reduction of AT III and thrombosis as a result, or AT III deficiency due to its consumption. However, such low levels are allowed to suggest a primary subclinical hereditary deficiency of AT III.
The presence of hereditary thrombophilia is not known to be a direct cause of thrombotic diseases. In this patient there is a combination of a hereditary predisposition to thrombosis from acquired risk factors of venous thromboembolism: obesity and hyperhomocysteinemia, which may have led to the implementation of genetic disorders [10].
Thus, in patients with multifocal thrombosis at a young age it is necessary to exclude the presence of congenital and acquired thrombophilia. The presence of congenital and acquired thrombophilia should be considered as an additional risk factor for cardiovascular disease.
Conflict of interest
The work was done in the framework of the state task, Pirogov Russian National Research Medical University (state registration number of the research work АААА-А18-118040390145-2).
Acknowledgments
To Vladimir Andreevich Lazarev, therapist at the V. M. Buyanov Moscow City Clinical Hospital, for his great contribution in the layout of illustrative material.
References
- 1.Raskob G., Angchaisuksiri P., Blanco A., Buller H., Gallus A., Hunt B. Thrombosis: a major contributor to the global disease burden. J Thromb Haemost. 2014;12:1580–1590. doi: 10.1111/jth.12698. [DOI] [PubMed] [Google Scholar]
- 2.Heit J.A., Spencer F., White R. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3–14. doi: 10.1007/s11239-015-1311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu Y., Jia C., Shi Q., Zhu Y., Liu Y. Hyperhomocysteinemia in men with a reproductive history of fetal neural tube defects: three case reports and literature review. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000013998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morales Borges R.H., Pagan Lugo M.A., Martínez Osorio G.S., Ramírez B.N., Carlo Torres S.E., González M.J. Puerto Ricans show higher prevalence of MTHFR C677T and A1298C mutations and higher risk of thrombophilia even without hyperhomocysteinemia: a preliminary survey. Ann Rev Res. 2019;4:1–7. [Google Scholar]
- 5.Vossen C.Y., Conard J., Fontcuberta J., Makris M., Van Der Meer F.J., Pabinger I. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT) J Thromb Haemost. 2005;3:459–464. doi: 10.1111/j.1538-7836.2005.01197.x. [DOI] [PubMed] [Google Scholar]
- 6.Khan S., Dickerman J.D. Hereditary thrombophilia. Thromb J. 2006;4:15. doi: 10.1186/1477-9560-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens S.M., Woller S.C., Bauer K.A., Kasthuri R., Cushman M., Streiff M. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154–164. doi: 10.1007/s11239-015-1316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soare A., Popa C. Deficiencies of proteins C, S and antithrombin and factor V Leiden and the risk of ischemic strokes (review) Med Life. 2010;3:235–238. [PMC free article] [PubMed] [Google Scholar]
- 9.Wypasek E., Corral J., Alhenc-Gelas M., Sydor W., Iwaniec T., Celińska-Lowenhoff M. Genetic characterization of antithrombin, protein C, and protein S deficiencies in Polish patients. Pol Arch Intern Med. 2017;127:512–523. doi: 10.20452/pamw.4045. [DOI] [PubMed] [Google Scholar]
- 10.Heit J.A. Thrombophilia: clinical and laboratory assessment and management. In: Kitchens C.S., Konkle B.A., Kessler C.M., editors. Consultative hemostasis and thrombosis. third edition. Elsevier Saunders; Philadelphia: 2013. pp. 205–239. [Google Scholar]