-
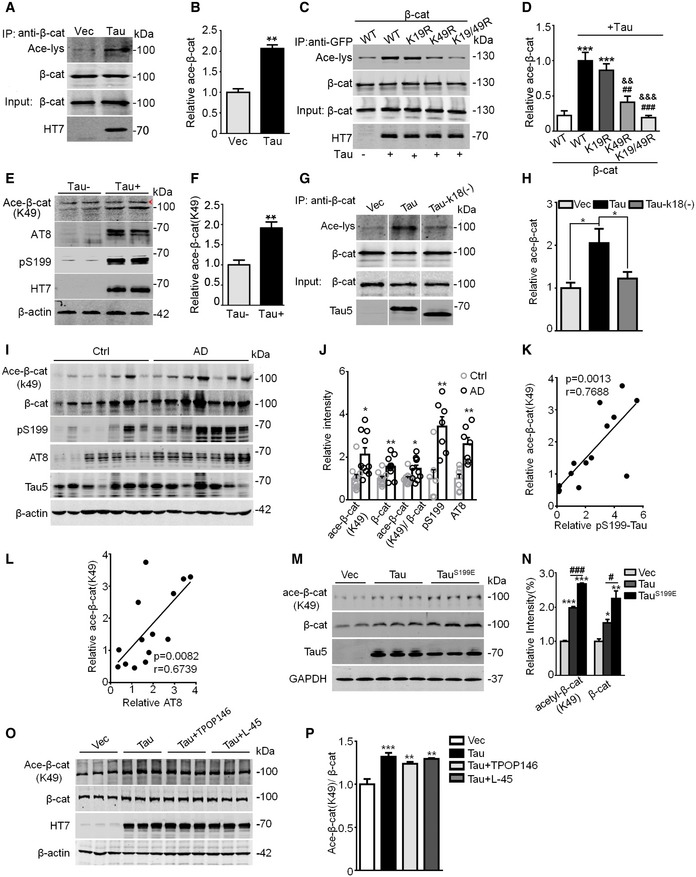
A, B
Tau increased β‐catenin acetylation (Ace‐β‐cat) measured by immunoprecipitation using anti‐β‐cat, or Western blotting using anti‐β‐cat, anti‐acetylated lysine (Ace‐lys), and HT7 (probes specifically human total Tau). HEK293 cells transiently transfected with wild‐type Tau40 (Tau) or the empty vector (Vec) (n = 3 from three independent experiments).
-
C, D
β‐cat mutation at K49 (K49R) but not K19 (K19R) abolished Tau‐induced acetylation. HEK293 cells were co‐transfected with eGFP‐β‐cat (WT, or K19R, or K49R, or K19R/K49R) and Tau or the empty vector for 48 h, and then immunoprecipitated using anti‐GFP and Western blotting using anti‐β‐cat, anti‐Ace‐lys, and HT7 (n = 3 from three independent experiments).
-
E, F
The increased β‐cat (K49) acetylation was detected in the hippocampal extracts of human Tau transgenic mice (Tau+) compared with the endogenous Tau knockout mice (Tau−), measured by Western blotting using acetylation site‐specific antibody (Ace‐β‐cat‐K49); arrowhead in panel (E) denoted non‐specific band identified by molecular weight. The remarkably increased levels of phosphorylated Tau at Ser199 (pS199) and AT8 epitopes were detected in Tau+ mice (at least three mice per group were used).
-
G, H
K18 deletion of Tau (Tau‐k18(−)) attenuated Tau‐induced β‐cat acetylation. HEK293 cells were transfected with empty Vec, or Tau40 or Tau‐k18(−) for 48 h, and the ace‐β‐cat was measured by immunoprecipitation using anti‐β‐cat and Western blotting using anti‐ace‐lys, anti‐β‐cat, and Tau5 (detecting total Tau; n = 3 from three independent experiments).
-
I–L
The increased total and Ace‐β‐cat (K49) levels positively correlated with increased phosphorylated Tau pS199 and AT8 as detected by Western blotting in the hippocampal extracts of AD patients compared with age‐matched controls (
n = 10 each group, detailed information for human brain samples was shown in
Appendix Table S1). Pearson's analysis was used for correlation analysis (K and L).
-
M, N
Expressing pseudo‐phosphorylated Tau (TauS199E) further increased the K49 acetylation of β‐catenin. N2a cells were transiently transfected with Vec, Tau40, or TauS199E for 48 h, levels of K49‐acetylated β‐cat, total β‐cat, and total Tau were detected by Western blotting (n = 3 biological replicates each group).
-
O, P
Inhibiting acetyltransferases CBP/P300 by TPOP146 (134 nM) or PCAF by L‐45 (126 nM) for 24 h did not significantly decrease the K49‐acetylated β‐cat level in Tau‐overexpressing N2a cells (n = 3 biological replicates each group).
Data information: Data are presented as mean ± SEM; *
P <
0.05; **
P <
0.01; ***
P <
0.001 vs. Vec (B, N and P), or vs. β‐cat WT (D), or vs. Tau− (F), or vs. Tau (H), or vs. Ctrl (J);
#
P <
0.05;
##
P <
0.01;
###
P <
0.001 vs. β‐cat WT plus Tau (D), or vs. Tau (N);
&&
P <
0.01;
&&&
P <
0.001 vs. β‐cat K19R plus Tau (D); the absence of asterisk indicates that the difference is not significant. Data were analyzed by Student's
t‐test (B, F and J) or one‐way ANOVA (D, H, N and P).
Source data are available online for this figure.