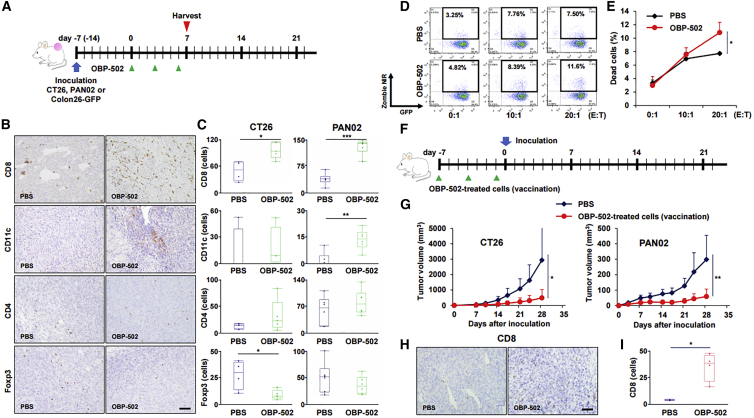
Figure 3.
Recruitment of CD8-Positive Lymphocytes to Tumors and Development of Acquired Antitumor Immunity Mediated by OBP-502
(A) Study protocol. In brief, CT26 or PAN02 subcutaneous tumors, intratumorally treated with OBP-502 (1 × 109 PFUs) or PBS three times each week, were harvested at 7 days after initiation of treatment for immunohistochemical staining. (B) Representative figures of immunohistochemical staining for CD8, CD11c, CD4, and Foxp3 in CT26 tumor tissues. Scale bar, 100 μm. (C) Median number of TILs expressing CD8, CD11c, CD4, and Foxp3 was statistically assessed from five selected fields. *p < 0.05, **p < 0.005, ***p < 0.001. (D) Representative figures of FACS for CTL assay in which cytotoxicity of CD8-positive lymphocytes (effector) harvested from the spleen of mice treated with OBP-502 (1 × 109 PFUs) or PBS on Colon26-GFP cells (target) was analyzed at different ratios of effector/target of 0:1, 10:1, and 20:1. Cells in the area surrounded by the black border are dead Colon26-GFP cells. (E) Percentage of dead cells in CTL assay was statistically assessed between PBS and OBP-502 (n = 3). *p < 0.05. (F) Protocol for the vaccination study. In brief, CT26 or PAN02 cells treated with OBP-502 (1,000 MOI) for 3 days were administered subcutaneously into the flank of BALB/c or C57/BL6 mice on days −7, −4, and −1 for vaccination, and CT26 or PAN02 cells (1 × 105 cells) were inoculated subcutaneously on day 0. (G) Tumor volume was monitored until day 28 and compared between PBS and vaccinated mice. *p < 0.05, **p < 0.005. (H) Representative figures of immunohistochemical staining for CD8-positive TILs in tumor tissues harvested 35 days after PAN02 inoculation in mice vaccinated with OBO-502-treated PAN02 cells or PBS. Scale bar, 100 μm. (I) Median number of TILs expressing CD8 was statistically assessed from five selected fields. *p < 0.05. E:T, effector/target.