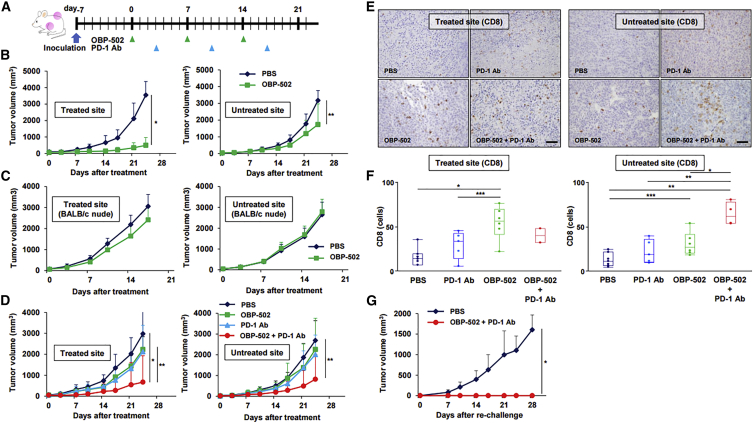
Figure 5.
Abscopal Effects of Combination Therapy in a Bilateral Subcutaneous Tumor Model
(A) Study protocol. In brief, in the bilateral subcutaneous tumor model, one side was treated with OBP-502 intratumorally (1 × 109 PFUs) three times each week, and the other side was left untreated with OBP-502. PD-1 Ab was administered intraperitoneally (1st: 20 mg/kg, 2nd and 3rd: 10 mg/kg) three times each week. (B) Volume of CT26 tumors treated with PBS or OBP-502 was monitored separately at the OBP-502-treated site and untreated site until day 24 (n = 7). *p < 0.001, **p < 0.05. (C) The same experiment shown in (B) was performed using BALB/c nude mice, and tumor volume was monitored until day 21 (n = 7). (D) Volume of CT26 tumors treated with PBS, monotherapy with OBP-502 or PD-1 Ab, or combination of both was monitored separately at the OBP-502-treated site and untreated site until day 24 (n = 6). *p < 0.005, **p < 0.05. (E) Representative figures for each treatment group of immunohistochemical staining for CD8-positive TILs in OBP-502-treated tumor tissues and untreated tumor tissues harvested 28 days after initiation of treatment. Scale bar, 100 μm. (F) Median number of CD8-positive TILs in OBP-502-treated tumor tissues and untreated tumor tissues was statistically assessed from five selected fields. *p < 0.005, **p < 0.001, ***p < 0.05. (G) Four mice rendered tumor free (OBP-502-treated site) by combination therapy with OBP-502 and PD-1 Ab in the experiment shown in (D) were re-challenged with CT26 inoculation (5 × 105 cells). Four naive mice were inoculated with CT26 cells (5 × 105 cells) and used as controls. Tumor volume was monitored until day 28. *p < 0.005.