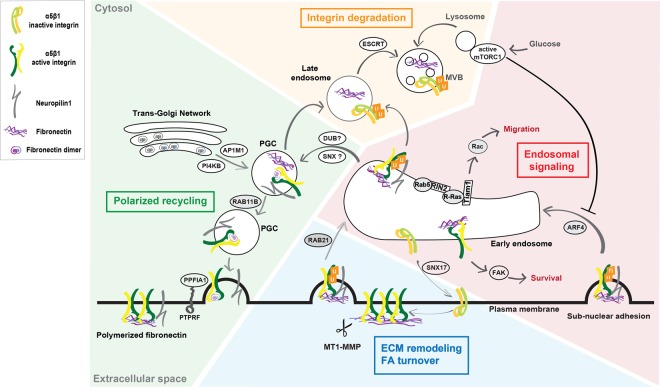
Figure 1. Active integrin internalization and trafficking pathways.
Upon MMP-dependent cleavage of polymerized FN fibrils, the endocytic receptor NRP1 interacts on the cell surface with FN fragment-bound ubiquitinated α5β1 active-integrin that is internalized within early endosomes (EEs) through either Rab21, or Rab5, or Afr4 small GTPase, depending on the internalization site and cell type (see text and Figure 2). In EEs, active α5β1 integrin may activate different signaling pathways such as: (i) Rac1 to promote integrin-dependent cell spreading and migration on FN; (ii) FAK to suppress anoikis in normal cells or promote cancer cell anchorage-independent growth. Moreover, in Rab25 overexpressing ovarian cancer cells Arf4-dependent active α5β1 integrin is required for correct lysosome positioning and activation of mTORC1, whose kinase activity is also regulated by glucose levels. From the EE compartment, inactive integrins can move back to the plasma membrane via sorting nexin 17 (SNX17) and the retriever complex [124]. A fraction of FN fragment-bound active α5β1 integrins is sorted into multivesicular bodies (MVBs) and degraded into lysosomes; this degradative fate depends on α5β1 integrin ubiquitination and on endosomal sorting complexes required for transport (ESCRT) protein complex. Another fraction of FN fragment-bound active α5β1 integrin instead traffics to PGCs; it is still unclear if this latter step relies on deubiquitinase (DUB) and/or SNX activities. It is posited that, in PGCs, endocytosed old FN may be separated from active α5β1 integrin that would then be free and able to bind freshly synthesized FN originating from TGN cisternae in a PI4KB and AP-1A-dependent manner. Through a RAB11B-dependent endosomal recycling pathway, vesicles containing newly synthesized FN-bound active α5β1 integrins are next directed to the basolateral side of the cell surface, where the PTPRF/PPFIA1 complex support their docking, likely via PPFIA1-mediated interaction with the β1 integrin subunit cytodomain.