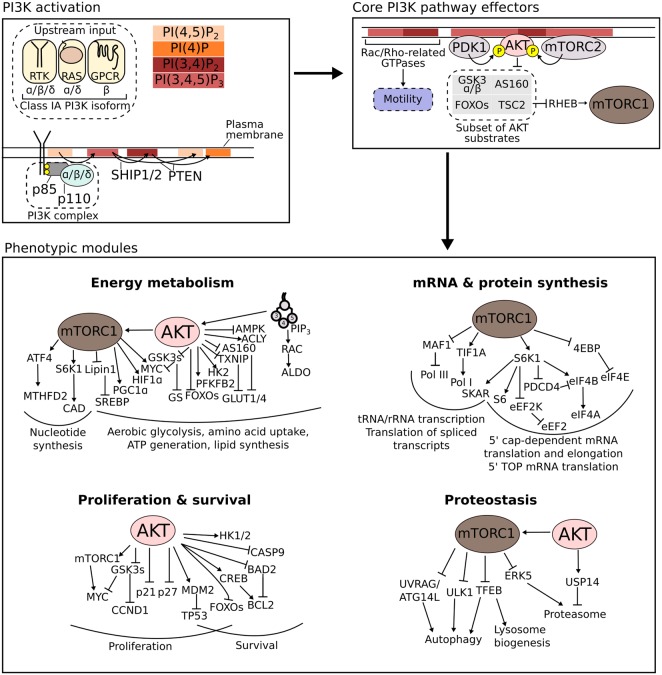
Figure 1. An overview of class IA PI3K signalling.
The class IA PI3K heterodimer consists of one of three different catalytic subunits (p110α/β/γ) and one of five different regulatory subunits (p85α/β, p55α/γ, p50α). Its activation involves recruitment to the plasma membrane where its substrate, the phosphoinositide PI(4,5)P2 is located. A common mechanism of activation involves the binding of the regulatory p85 subunit to phosphotyrosine residues on receptor tyrosine kinases (RTKs) or their associated adaptor proteins. The catalytic subunits of PI3Kα and PI3Kδ can also interact and be activated by RAS. This is not the case for PI3Kβ which instead can be activated by other small GTPases downstream of G protein-coupled receptors (GPCRs). The immediate output of class IA PI3K activation is the generation of the second messenger PI(3,4,5)P3 and its derivative PI(3,4)P2. These are detected by proteins with specialised phosphoinositide-binding domains, with AKT representing one of the most studied examples. Through AKT-dependent and -independent effectors, the PI3K pathway orchestrates an array of diverse phenotypic modules whose execution is highly context-dependent [8]. Negative feedback loops and cross-talk with other pathways are omitted for clarity. For comprehensive PI3K signalling reviews, the reader is referred to Ref. [1,2,4,113].