Figure 3: APOE isoforms and Aβ aggregation and clearance.
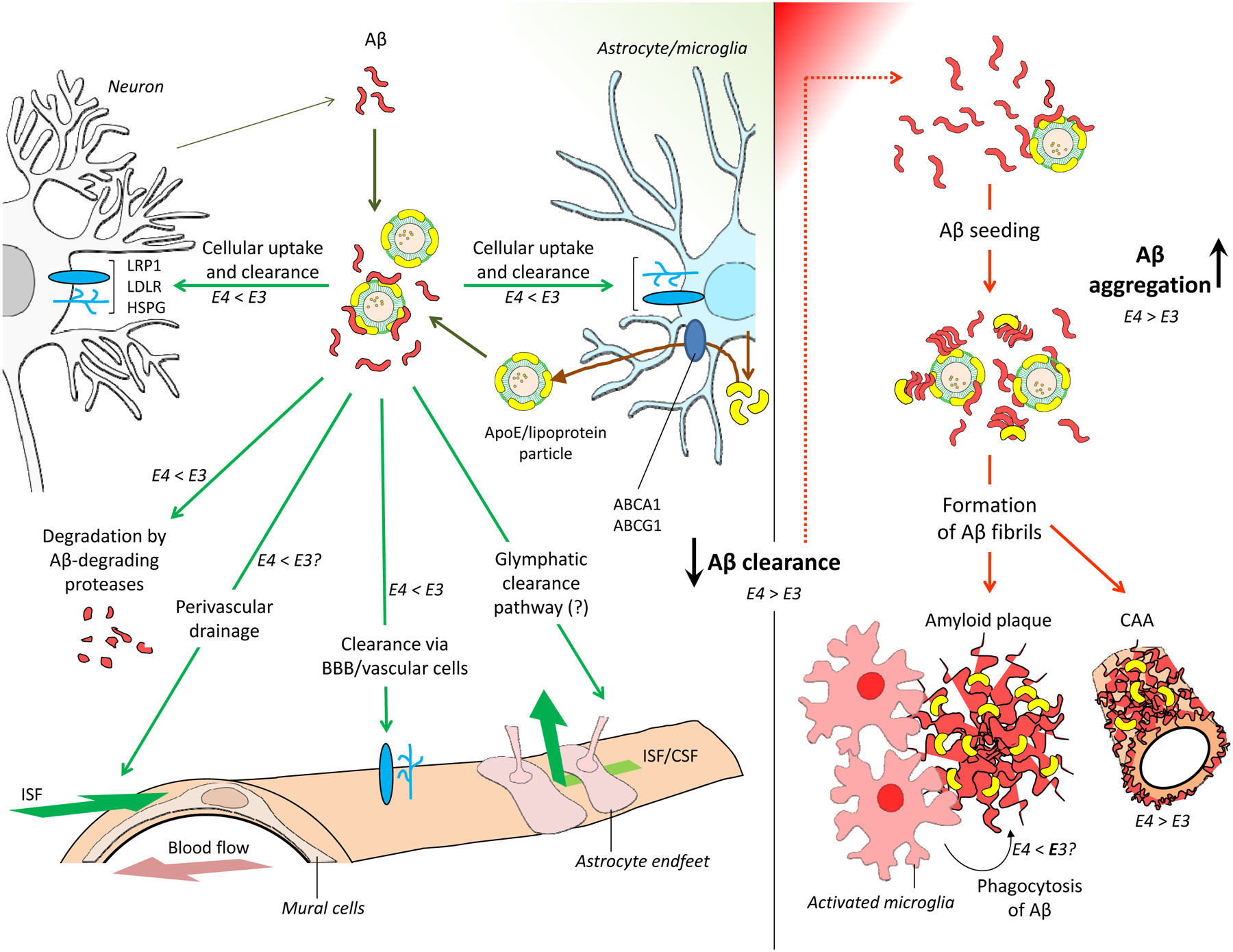
a | Amyloid-β (Aβ) production and clearance pathways. Aβ is produced primarily in neurons through proteolytic cleavage of amyloid precursor protein (grey arrow). Aβ is then removed from the brain by multiple Aβ clearance pathways (green boxes), including cellular uptake and subsequent degradation, enzymatic degradation, clearance through the blood–brain barrier (BBB), and clearance via interstitial fluid (ISF) bulk flow and, potentially, the glymphatic pathway. LDL receptor-related protein 1 (LRP1), LDL receptor (LDLR) and heparan sulfate proteoglycan (HSPG) are major APOE receptors that mediate cellular uptake of Aβ. Apolipoprotein E (APOE) is produced and lipidated primarily by astrocytes (brown arrow). A sub-pool of APOE lipoprotein particles interacts with soluble Aβ released into the brain interstitial fluid by neurons. b | Insufficient Aβ clearance from the brain leads to Aβ accumulation. This accumulation initiates Aβ oligomerization and accelerates subsequent aggregation and fibrillogenesis, leading to deposition of insoluble Aβ in the brain parenchyma (amyloid plaques) and in the vascular wall (cerebral amyloid angiopathy). APOE promotes the formation of Aβ fibrils by accelerating the initial seeding or nucleation of Aβ peptides. APOE can influence Aβ clearance and aggregation, either directly or indirectly, in an isoform-dependent manner. The relative abilities of APOE3 and APOE4 to promote each pathway are indicated alongside the arrows. ABCA1, ATP-binding cassette sub-family A member 1; ABCG1, ATP-binding cassette sub-family G member 1; CSF, cerebrospinal fluid.
