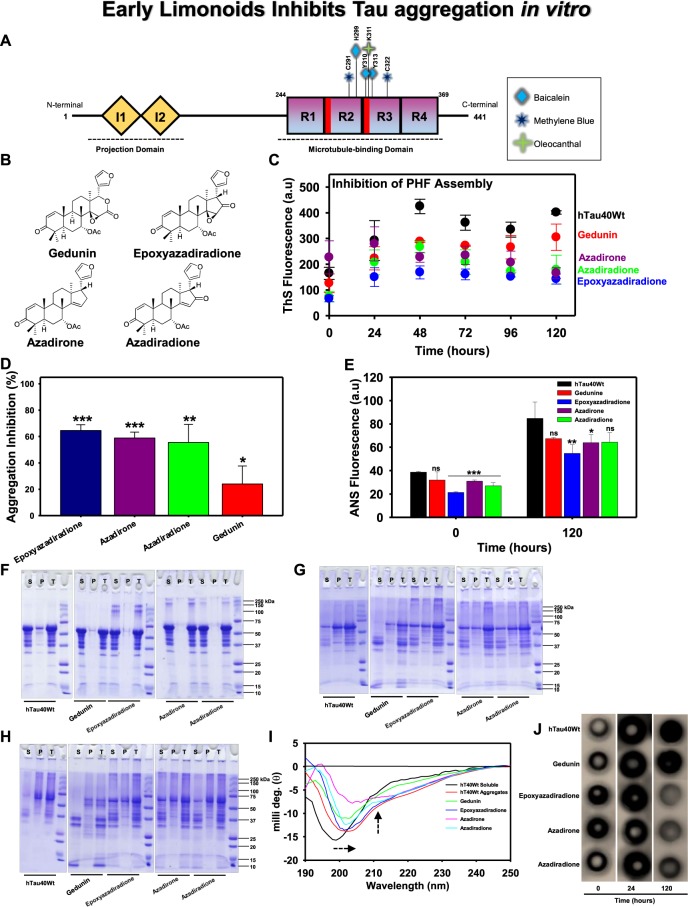
Figure 1.
The aggregation inhibition of Tau by basic limonoids. (A) Diagrammatic representation of full-length Tau comprising 441 amino acids with two inserts towards N-terminal and four repeats towards C-terminal. The inserts functions as projection domain, which serves to separate microtubule bundles. The four repeats act as microtubule-binding domain and helps in tubulin polymerization into microtubules. AD triggers pathological modifications in repeat region, leading to the loss of Tau affinity for microtubule. This causes destabilization of microtubule and aggregation of Tau. The bar diagram depicts the binding sites for various small molecules such as Baicalein, Methylene blue and Oleocanthal interacting with Tau. (B) Structure of the basic limonoids isolated from the fruit coat of Azadirachta indica. (C,D) Inhibition of full-length Tau aggregation by basic limonoids was analysed by ThS fluorescence assay which shows the potency of epoxyazadiradione over other limonoids, which was also represented as rate of inhibition in terms of percentage. (E) ANS fluorescence revealed the change of hydrophobicity content in Tau at time intervals of 0 and 120 hours, according to which epoxyazadiradione have persistently low hydrophobicity when compared to control and other limonoids. (F) The SDS-PAGE analysis showed the formation of higher order aggregates at 0 hour by epoxyazadiradione, azadirone and azadiradione, whereas full-length Tau, the control shows no signature of aggregation. The sedimentation assay revealed the presence of these aggregates in supernatant (S) and not in the pellet (P) which indicated that these were soluble aggregates. (G) After 24 hours of incubation the full-length Tau aggregated and this phenomenon was observed in control and test. This was witnessed by Tau present in the pellet (P) fraction obtained after sedimentation. Tau exhibited extensive degradation in presence of gedunin, whereas other limonoids showed Tau aggregation. (H) At 120 hours the Tau was completely aggregated and separated in the pellet (P). Gedunin exhibited mostly the degradation of soluble Tau although aggregation was also evidenced. Epoxyazadiradione, azadirone and azadiradione also exhibited Tau aggregation at varied extent. (I) The CD spectra of native Tau showed random coil conformation, whereas on aggregation the spectra signified β-sheet conformation. Similarly, in presence of limonoids the change in Tau conformation to β-sheet was observed. The basic limonoids exhibited difference in the spectrum intensity and shift. This could be due to the variation in the structure of limonoids which led Tau to attain varied conformations. (J) The aggregation of Tau was probed by K9JA reveals the initial aggregation by limonoids at 0 and 24 hours. Later at 120 hours of incubation shows the fully aggregated Tau in absence of limonoids. Epoxyazadiradione showed more potential in inhibiting aggregation, followed by Azadirone and Azadiradione. Gedunin had poor effects in preventing aggregates formation. The graph was plotted using SigmaPlot 10.0, from Systat Software, Inc., San Jose California USA, www.systatsoftware.com.