Figure 6. CCR engineering provides a general strategy for avoiding on-target off-tumor toxicity.
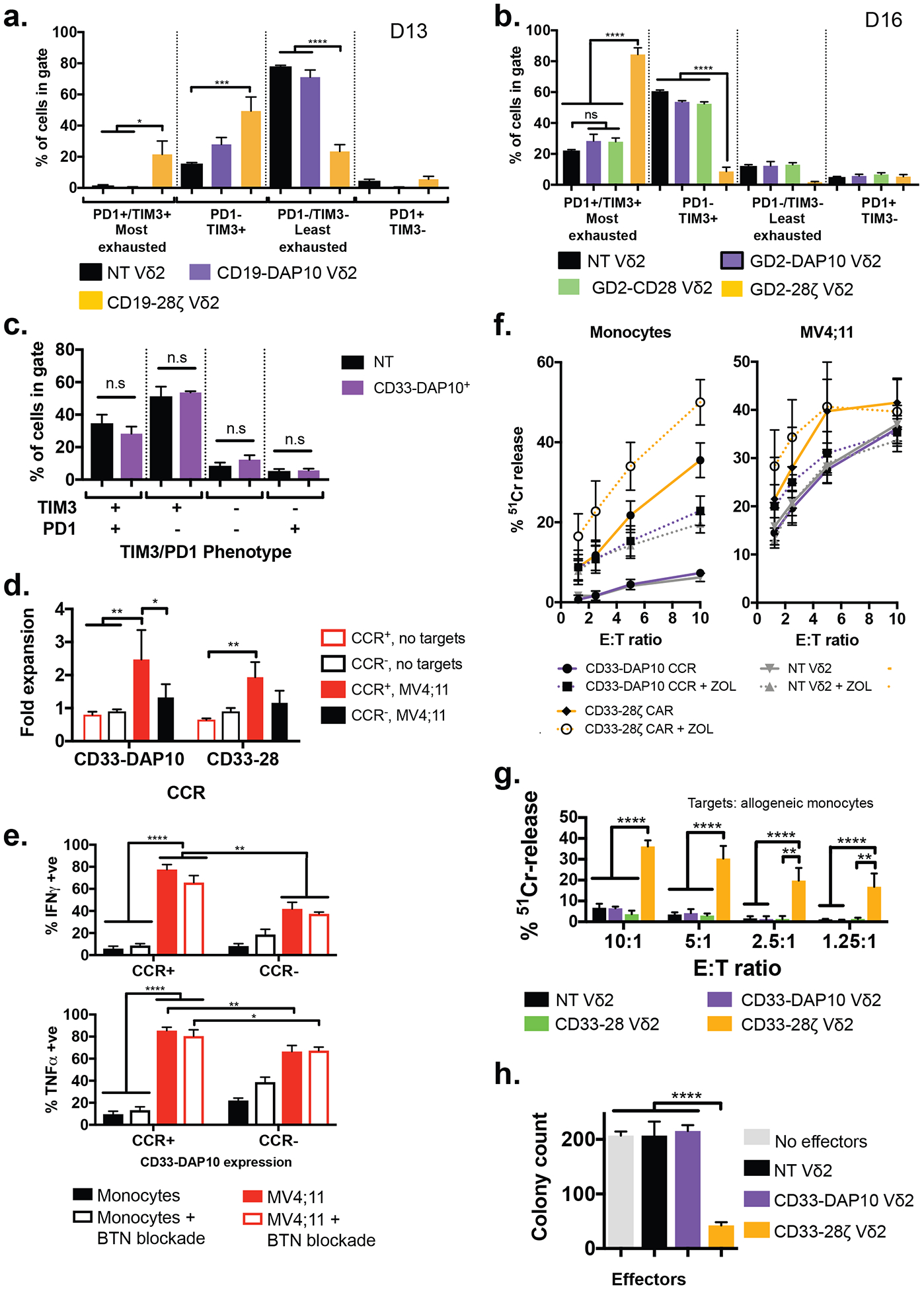
A) Exhaustion status as defined by TIM3 and PD1 expression of Vδ2+ cells expressing a CD19–28ζ CAR or a CD19-DAP10 CCR (identical aside from the endodomain configuration), compared to donor-matched untransduced cells (n = 3 donors, values mean ± SEM). Cells were analyzed 13 days after initial stimulation, 8 days post transduction. * p <0.033, ** p <0.0021, *** p<0.0002, **** p<0.0001 by one-way ANOVA with Sidak’s correction. Example gating is shown in fig. S15.
B) Exhaustion status defined as in (A) for Vδ2+ cells expressing either the GD2-CD28-CCR (n = 3), GD2-DAP10-CCR (n = 3, data from Fisher et al(37)) or GD2–28ζ CAR (n = 3), compared to untransduced controls (n = 18) cultured for 16 days following initial stimulation (11 days post transduction). * p <0.033, ** p <0.0021, *** p<0.0002, **** p<0.0001 by one-way ANOVA with Sidak’s correction.
C) Exhaustion status for CD33-DAP10 Vδ2+ cells as in (A) shows no significant difference to that of untransduced Vδ2+ cells after 16 days of culture (11 days post transduction, n = 3, values represent mean ± SEM compared by one-way ANOVA with Sidak’s correction)
D) Fold expansion of Vδ2+ cells ± CD33-DAP10 or CD33-CD28 CCRs in the presence of irradiated MV4;11 AML cells. MV4;11 induced significant proliferation only in CCR+ cells (p = 0.0025 and p = 0.0097, respectively). CD33-DAP10 expression confers significant (p = 0.016) AML-induced proliferative benefit over untransduced controls. (2-way ANOVA with Tukey’s multiple comparisons correction, bars show mean ±SEM of 3 independent donors).
E) Production of IFNγ and TNFα by DAP10-CCR+/− Vδ2+ γδT cells co-cultured overnight with either MV4;11 or allogeneic monocytes at a 1:1 effector:target ratio, in the presence or absence of butyrophillin 3A1 blockade. When in contact with MV4;11, CCR+ Vδ2 express significantly more of both cytokines (p = 0.008 for TNFα and 0.002 for IFNγ). Cytokine production by DAP10-CCR+ cells is significantly higher when in contact with MV4;11 than it is when in contact with monocytes (p<0.0001). Blockade of butyrophillin did not affect cytokine production. Bars are mean ± SEM of 3 independent donors and significance determined using 2-way ANOVA with Sidak’s correction.
F) 4-hour 51Cr release assay detecting killing of allogeneic monocytes or MV4;11 by non-transduced Vδ2 γδT cells or those expressing either CD33-DAP10 CCR or CD33–28ζ CAR, in the presence or absence of zoledronic acid. Zoledronic acid treatment enhanced killing of monocytes by NT Vδ2 (p = 0.01) or CD33-DAP10 Vδ2 (p=0.0005) but not of MV4;11. CD33-DAP10 did not enhance cytotoxicity against either target, and CD33–28ζ significantly enhanced killing of monocytes (p<0.0001) but not MV4;11. Data shows mean ± SEM of 3–10 independent donors, analysis by 2-way ANOVA with Sidak’s correction.
G) CD33-DAP10 (n=6) or CD33-CD28 (n=3) Vδ2+ cells do not kill healthy allogeneic monocytes and more than untransduced Vδ2 (n = 9), unlike CD33–28ζ CAR-transduced Vδ2+ cells (n=6) (comparisions by 2-way ANOVA with Tukey’s multiple comparison correction). Data is from 4h 51Cr release assays at the range of effector:target (E:T) ratios shown.
H) Myeloid colony formation assay for Vδ2+ cells expressing either CD33–28ζ or CD33-DAP10 and co-cultured overnight with healthy bone marrow. Only CD33–28ζ led to significant reduction in myeloid colony formation, demonstrating the lack of CD33-DAP10 Vδ2+ cell toxicity against healthy myeloid progenitors. Results for 3 independent γδT cell donors, mean ± SEM is shown, compared by one-way ANOVA with Sidak’s correction, ****p<0.0001.
